Ethyl cyanoglyoxylate-2-oxime
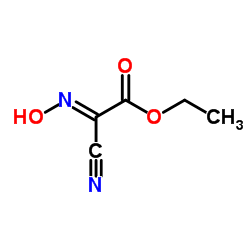
Ethyl cyanoglyoxylate-2-oxime structure
|
Common Name | Ethyl cyanoglyoxylate-2-oxime | ||
|---|---|---|---|---|
| CAS Number | 3849-21-6 | Molecular Weight | 142.11 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 248.9±23.0 °C at 760 mmHg | |
| Molecular Formula | C5H6N2O3 | Melting Point | 130-132 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 104.3±22.6 °C | |
Use of Ethyl cyanoglyoxylate-2-oximeEthyl 2-cyano-2-hydroxyiminoacetate is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | Ethyl cyanoglyoxylate-2-oxime |
|---|---|
| Synonym | More Synonyms |
| Description | Ethyl 2-cyano-2-hydroxyiminoacetate is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog | |
| In Vitro | 高效偶联添加剂,是HOBt的更安全、不爆炸的替代品。 |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 248.9±23.0 °C at 760 mmHg |
| Melting Point | 130-132 °C(lit.) |
| Molecular Formula | C5H6N2O3 |
| Molecular Weight | 142.11 |
| Flash Point | 104.3±22.6 °C |
| Exact Mass | 142.037842 |
| PSA | 82.68000 |
| LogP | 0.35 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.496 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S22-S24/25-S36/37 |
| RIDADR | 3276 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2928000090 |
|
~93% 
Ethyl cyanoglyo... CAS#:3849-21-6 |
| Literature: Synthesis, , # 1 p. 46 - 48 |
|
~% 
Ethyl cyanoglyo... CAS#:3849-21-6 |
| Literature: Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), , # 2 p. 747 - 750 |
|
~% 
Ethyl cyanoglyo... CAS#:3849-21-6 |
| Literature: Annales de Chimie (Cachan, France), , vol. <7> 1, p. 479,499 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Carboxymethylcellulose-tetrahydrocurcumin conjugates for colon-specific delivery of a novel anti-cancer agent, 4-amino tetrahydrocurcumin.
Eur. J. Pharm. Biopharm. 88(2) , 351-60, (2014) Several curcumin derivatives are now becoming increasingly of interest because of their bioactive attributes, especially their action as antioxidants and anti-carcinogenic activities. Tetrahydrocurcum... |
|
|
COMU-Safer and More Efficient Peptide Coupling Reagent. Junkers M. COMU-Safer and More Efficient Peptide Coupling Reagent. Aldrich Chemfiles 10(1) , (2010)
|
|
|
Route to three-dimensional fragments using diversity-oriented synthesis.
Proc. Natl. Acad. Sci. U. S. A. 108(17) , 6799-804, (2011) Fragment-based drug discovery (FBDD) has proven to be an effective means of producing high-quality chemical ligands as starting points for drug-discovery pursuits. The increasing number of clinical ca... |
| OxiMa |
| Ethyl isonitrosocyanoacetate |
| EINECS 223-351-3 |
| Ethyl cyanoximidoacetate |
| Ethyl Cyano(hydroxyimino)acetate |
| Acetic acid, 2-cyano-2-(hydroxyimino)-, ethyl ester, (2E)- |
| ETHYL OXIMINOCYANOACETATE |
| ETHYL CYANOGLYOXYLATE OXIME |
| ETHYL CYANOGLYOXALATE-2-OXIME |
| Ethyl Cyanoglyoxylate-2-Oxime |
| Cyano(hydroxyimino)acetic Acid Ethyl Ester |
| Ethyl (Hydroxyimino)cyanoacetate |
| Oxyma Pure |
| Ethyl (2E)-cyano(hydroxyimino)acetate |
| 2-Ethyloximinocyanocaetate |
| Ethyl Cyanoglyoxalate Oxime |
| MFCD00002112 |
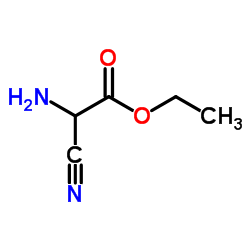 CAS#:32683-02-6
CAS#:32683-02-6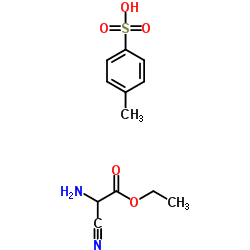 CAS#:37842-58-3
CAS#:37842-58-3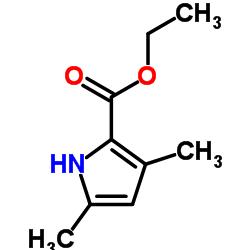 CAS#:2199-44-2
CAS#:2199-44-2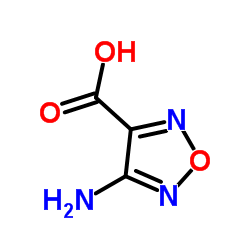 CAS#:78350-50-2
CAS#:78350-50-2 CAS#:68462-61-3
CAS#:68462-61-3 CAS#:6829-41-0
CAS#:6829-41-0 CAS#:36231-81-9
CAS#:36231-81-9 CAS#:33235-31-3
CAS#:33235-31-3 CAS#:21190-16-9
CAS#:21190-16-9 CAS#:113120-63-1
CAS#:113120-63-1
