Antipain
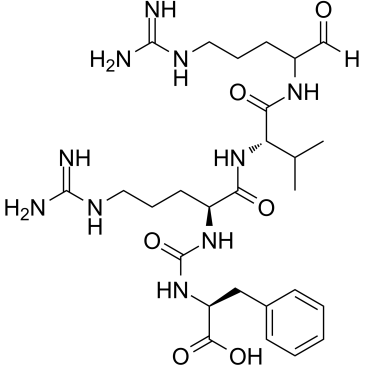
Antipain structure
|
Common Name | Antipain | ||
|---|---|---|---|---|
| CAS Number | 37691-11-5 | Molecular Weight | 604.70200 | |
| Density | 1.38 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C27H44N10O6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of AntipainAntipain, a protease inhibitor isolated from Actinomycetes. Antipain inhibits N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced transformation and increases chromosomal aberrations[1][2]. |
| Name | antipain hydrochloride dihydrate |
|---|---|
| Synonym | More Synonyms |
| Description | Antipain, a protease inhibitor isolated from Actinomycetes. Antipain inhibits N-methyl-N'-nitro-N-nitrosoguanidine (MNNG)-induced transformation and increases chromosomal aberrations[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.38 g/cm3 |
|---|---|
| Molecular Formula | C27H44N10O6 |
| Molecular Weight | 604.70200 |
| Exact Mass | 604.34500 |
| PSA | 277.50000 |
| LogP | 2.64660 |
| Index of Refraction | 1.631 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
A kinetic characterization of the gill V(H(+))-ATPase in juvenile and adult Macrobrachium amazonicum, a diadromous palaemonid shrimp.
Comp. Biochem. Physiol. B Biochem. Mol. Biol. 181 , 15-25, (2015) Novel kinetic properties of a microsomal gill V(H(+))-ATPase from juvenile and adult Amazon River shrimp, Macrobrachium amazonicum, are described. While protein expression patterns are markedly differ... |
|
|
Inhibition of cathepsin proteases attenuates migration and sensitizes aggressive N-Myc amplified human neuroblastoma cells to doxorubicin.
Oncotarget 6 , 11175-90, (2015) Neuroblastoma arises from the sympathetic nervous system and accounts for 15% of childhood cancer mortality. Amplification of the oncogene N-Myc is reported to occur in more than 20% of patients. Whil... |
|
|
Lysosomal membrane permeabilization: carbon nanohorn-induced reactive oxygen species generation and toxicity by this neglected mechanism.
Toxicol. Appl. Pharmacol. 280(1) , 117-26, (2014) Understanding the molecular mechanisms responsible for the cytotoxic effects of carbon nanomaterials is important for their future biomedical applications. Carbon nanotubular materials induce the gene... |
| MFCD00135959 |
| Phe-co-Arg-Val-L-Arg-h |
| N2-[[(1-carboxyphenethyl)amino]carbonyl]-L-arginyl-N-[4-[(aminoiminomethyl)amino]-1-formylbutyl]-L-valinamide |
| EINECS 253-631-0 |
| 4-((aminoiminomethyl)amino)-1-formylbutyl) |
| N2-[[(1-Carboxy-2-phenylethyl)amino]carbonyl]-L-arginyl-N-[4-[(aminoiminomethyl)amino]-1-formylbutyl]-L-valinamide |