5-vinyluracil
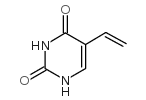
5-vinyluracil structure
|
Common Name | 5-vinyluracil | ||
|---|---|---|---|---|
| CAS Number | 37107-81-6 | Molecular Weight | 138.12400 | |
| Density | 1.332g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C6H6N2O2 | Melting Point | >300ºC (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | 5-ethenyl-1H-pyrimidine-2,4-dione |
|---|---|
| Synonym | More Synonyms |
| Density | 1.332g/cm3 |
|---|---|
| Melting Point | >300ºC (dec.)(lit.) |
| Molecular Formula | C6H6N2O2 |
| Molecular Weight | 138.12400 |
| Exact Mass | 138.04300 |
| PSA | 65.72000 |
| Appearance of Characters | Powder |
| Index of Refraction | 1.606 |
| Storage condition | -20°C Freezer |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | 26-36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933599090 |
| Precursor 4 | |
|---|---|
| DownStream 5 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
A method for the rapid preparation of 5-vinyluracil in high yield.
Nucleic Acids Res. 1(1) , 105-7, (1974) A method for the rapid preparation of the thymineanalogue, 5-vinyluracil, in 83% yield from 5-(1-hydroxyethyl)uracil via the methanesulphonyl ester is reported. |
|
|
Design and synthesis of the tumor-activated prodrug of dihydropyrimidine dehydrogenase (DPD) inhibitor, RO0094889 for combination therapy with capecitabine.
Bioorg. Med. Chem. Lett. 13(5) , 867-72, (2003) A series of tumor-activated prodrugs of the inhibitors of dihydropyrimidine dehydrogenase (DPD), an enzyme catabolizing 5-fluorouracil (5-FU: 4g), has been designed and synthesized. RO0094889 (11c) is... |
|
|
Incorporation of 5-substituted uracil derivatives into nucleic acids. The isolation and gamma-radiation-sensitivity of bacteriophage T3 containing the thymine analogue 5-vinyluracil.
Biochem. J. 187(1) , 257-60, (1980) Bacteriophage T3 was produced in a form that contained 32% of its normal DNA thymine residues replaced with 5-vinyluracil residues by infecting a thymine-requiring strain of Escherichia coli with phag... |
| 5-ethenyluracil |
| 5-vinyl-1H-pyrimidine-2,4-dione |
| MFCD00913267 |
| 5-ethenyl-2,4(1H,3H)-pyrimidinedione |
| 5-Vinyl-uracil |
| 5-ethenylpyrimidine-2,4-diol |
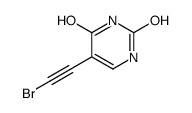 CAS#:77530-00-8
CAS#:77530-00-8 CAS#:7486-35-3
CAS#:7486-35-3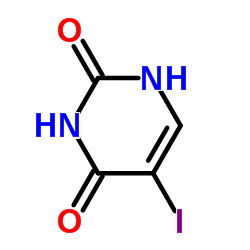 CAS#:696-07-1
CAS#:696-07-1 CAS#:108-05-4
CAS#:108-05-4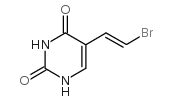 CAS#:69304-49-0
CAS#:69304-49-0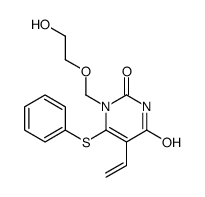 CAS#:125056-98-6
CAS#:125056-98-6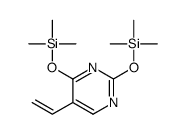 CAS#:55520-62-2
CAS#:55520-62-2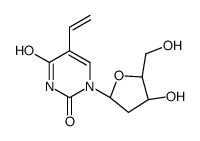 CAS#:55520-67-7
CAS#:55520-67-7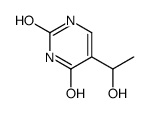 CAS#:39541-85-0
CAS#:39541-85-0