2-Hydroxyestradiol
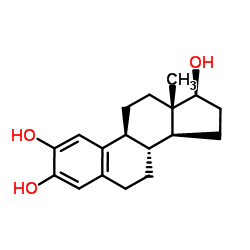
2-Hydroxyestradiol structure
|
Common Name | 2-Hydroxyestradiol | ||
|---|---|---|---|---|
| CAS Number | 362-05-0 | Molecular Weight | 288.38 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 481.5±45.0 °C at 760 mmHg | |
| Molecular Formula | C18H24O3 | Melting Point | 96-104°C | |
| MSDS | Chinese USA | Flash Point | 227.6±23.3 °C | |
| Symbol |

GHS08 |
Signal Word | Warning | |
Use of 2-Hydroxyestradiol2-Hydroxyestradiol, a metabolite of 17β-estradiol with minimal estrogenic activity, possesses antioxidant effects and reacts with DNA to form stable adducts and exerts genotoxicity[1][3]. |
| Name | 2-hydroxy-17β-estradiol |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Hydroxyestradiol, a metabolite of 17β-estradiol with minimal estrogenic activity, possesses antioxidant effects and reacts with DNA to form stable adducts and exerts genotoxicity[1][3]. |
|---|---|
| Related Catalog | |
| In Vitro | 2-Hydroxyestradiol induces phenotypical changes indicative of neoplastic transformation[1]. 2-Hydroxyestradiol (2-OHE2) is capable of in vitro transforming a HBEC, MCF-10F cell line, that is estrogen receptor-a (ERα) negative and ERβ positive[1]. 2-Hydroxyestradiol induces oxidative DNA damage and apoptosis in human mammary epithelial cells[3]. |
| In Vivo | 2-Hydroxyestradiol attenuated the development of obesity and improved endothelial function, decreased nephropathy, decreased the severity of diabetes, lowered arterial blood pressure, and reduced plasma cholesterol[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.5±45.0 °C at 760 mmHg |
| Melting Point | 96-104°C |
| Molecular Formula | C18H24O3 |
| Molecular Weight | 288.38 |
| Flash Point | 227.6±23.3 °C |
| Exact Mass | 288.172546 |
| PSA | 60.69000 |
| LogP | 3.53 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.622 |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Precursor 6 | |
|---|---|
| DownStream 4 | |
|
A methoxyflavonoid, chrysoeriol, selectively inhibits the formation of a carcinogenic estrogen metabolite in MCF-7 breast cancer cells.
J. Steroid Biochem. Mol. Biol. 118(1-2) , 70-6, (2010) A 17beta-estradiol (E(2)) is hydrolyzed to 2-hydroxy-E(2) (2-OHE(2)) and 4-hydroxy-E(2) (4-OHE(2)) via cytochrome P450 (CYP) 1A1 and 1B1, respectively. In estrogen target tissues including the mammary... |
|
|
Impact of induced fit on ligand binding to the androgen receptor: a multidimensional QSAR study to predict endocrine-disrupting effects of environmental chemicals.
J. Med. Chem. 48 , 5666-74, (2005) We investigated the influence of induced fit of the androgen receptor binding pocket on free energies of ligand binding. On the basis of a novel alignment procedure using flexible docking, molecular d... |
|
|
Norethisterone acetate alters coagulation gene expression in vitro in human cell culture.
Thromb. Res. 131(1) , 72-7, (2013) Both oestrogen and progestin and the route of administration have been implicated in cardiovascular and thromboembolic risk in post menopausal hormone users. Transdermal preparations have been reporte... |
| Estra-1,3,5(10)-triene-2,3,17β-triol |
| 2-Hydroxy-17b-estradiol |
| 2-hydroxy-17β-estradiol |
| 2-hydroxy-17beta-estradiol |
| MFCD00010490 |
| (17β)-Estra-1(10),2,4-triene-2,3,17-triol |
| Estra-1,3,5(10)-triene-2,3,17-triol, (17β)- |
| 2-Hydroxyestradiol |
| 2,3,17b-Trihydroxyestra-1,3,5(10)-triene |
| estra-1(10),2,4-triene-2,3,17-triol, (17β)- |
| Estra-1,3,5(10)-triene-2,3,17-β-triol |
| (17b)-Estra-1(10),2,4-triene-2,3,17-triol |
| (17β)-Estra-1,3,5(10)-triene-2,3,17-triol |
| 2-hydroxy-estradiol |
| 2-Oh-estradiol |
| Estra-1,3,5(10)-triene-2,3,17b-triol |
| 2-Hydroxy-17-estradiol |
 CAS#:362-07-2
CAS#:362-07-2 CAS#:50-28-2
CAS#:50-28-2![3-methoxy-13-methyl-6,7,8,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthrene-4,17-diol Structure](https://image.chemsrc.com/caspic/360/5976-65-8.png) CAS#:5976-65-8
CAS#:5976-65-8 CAS#:23463-05-0
CAS#:23463-05-0 CAS#:3589-91-1
CAS#:3589-91-1 CAS#:19590-55-7
CAS#:19590-55-7 CAS#:362-06-1
CAS#:362-06-1 CAS#:5976-67-0
CAS#:5976-67-0