9-Fluorenylmethyl carbazate
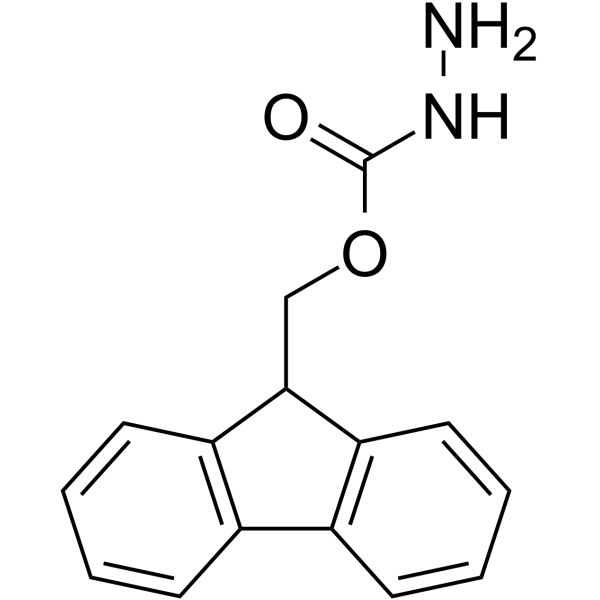
9-Fluorenylmethyl carbazate structure
|
Common Name | 9-Fluorenylmethyl carbazate | ||
|---|---|---|---|---|
| CAS Number | 35661-51-9 | Molecular Weight | 254.284 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 485.8±14.0 °C at 760 mmHg | |
| Molecular Formula | C15H14N2O2 | Melting Point | -170ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | 247.6±20.1 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 9-Fluorenylmethyl carbazate9-Fluorenylmethyl carbazate is used as a fluorophore reagent for a fluorimetric detection of glycans[1]. |
| Name | 9H-fluoren-9-ylmethyl N-aminocarbamate |
|---|---|
| Synonym | More Synonyms |
| Description | 9-Fluorenylmethyl carbazate is used as a fluorophore reagent for a fluorimetric detection of glycans[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 485.8±14.0 °C at 760 mmHg |
| Melting Point | -170ºC (dec.) |
| Molecular Formula | C15H14N2O2 |
| Molecular Weight | 254.284 |
| Flash Point | 247.6±20.1 °C |
| Exact Mass | 254.105530 |
| PSA | 64.35000 |
| LogP | 2.88 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.632 |
| InChIKey | YGCGPEUVGHDMLO-UHFFFAOYSA-N |
| SMILES | NNC(=O)OCC1c2ccccc2-c2ccccc21 |
| Storage condition | 2-8°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2928000090 |
|
~99% 
9-Fluorenylmeth... CAS#:35661-51-9 |
| Literature: Spiegel, Jochen; Mas-Moruno, Carlos; Kessler, Horst; Lubell, William D. Journal of Organic Chemistry, 2012 , vol. 77, # 12 p. 5271 - 5278 |
|
~% 
9-Fluorenylmeth... CAS#:35661-51-9 |
| Literature: WO2003/104196 A1, ; Page 36 ; WO 03/104196 A1 |
|
~% 
9-Fluorenylmeth... CAS#:35661-51-9 |
| Literature: Journal of Organic Chemistry, , vol. 37, # 22 p. 3404 - 3409 |
|
~% 
9-Fluorenylmeth... CAS#:35661-51-9 |
| Literature: Journal of Medicinal Chemistry, , vol. 55, # 14 p. 6502 - 6511 |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Synthesis and application of Fmoc-hydrazine for the quantitative determination of saccharides by reversed-phase high-performance liquid chromatography in the low and subpicomole range.
Anal. Biochem. 195 , 160, (1991) A new derivatization reagent, Fmoc-hydrazine, has been synthesized from the reaction of Fmoc-chloroformate with hydrazine as a precolumn fluorometric labeling reagent for reducing sugars such as gluco... |
|
|
Simple and sensitive multi-sugar-probe gut permeability test by high-performance liquid chromatography with fluorescence labelling.
J. Chromatogr. A. 730(1-2) , 99-105, (1996) Enteral intake of a mixture of inert, non-metabolic monosaccharide and disaccharide probes, followed by measurement of their urinary probe ratio, is a well known method to investigate gut permeability... |
|
|
A sensitive fluorescence reagent for the determination of aldehydes from alcoholic beverage using high-performance liquid chromatography with fluorescence detection and mass spectrometric identification.
Anal. Chim. Acta 636(1) , 95-104, (2009) A pre-column derivatization method for the sensitive determination of aldehydes using the tagging reagent 2-[2-(7H-dibenzo[a,g] carbazol-7-yl)-ethoxy] ethyl carbonylhydrazine (DBCEEC) followed by high... |
| Fmoc-hydrazide |
| Fmoc-hydrazine |
| (9H-Fluoren-9-yl)methyl hydrazinecarboxylate |
| Fmoc-NHNH2 |
| 9-Fluorenylmethyl Carbazate |
| [(9H-Fluoren-9-ylmethoxy)carbonyl]hydrazine |
| Carbazic Acid 9-Fluorenylmethyl Ester |
| 9H-Fluoren-9-ylmethyl hydrazinecarboxylate |
| Hydrazinecarboxylic acid, 9H-fluoren-9-ylmethyl ester |
| N-[(9H-fluoren-9-ylmethoxy)-carbonyl]-hydrazine |
| 9-Fluorenylmethylcarbazate |
 CAS#:250280-31-0
CAS#:250280-31-0
