4-Bromophenyl methyl sulfone
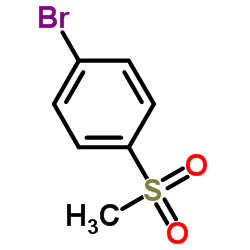
4-Bromophenyl methyl sulfone structure
|
Common Name | 4-Bromophenyl methyl sulfone | ||
|---|---|---|---|---|
| CAS Number | 3466-32-8 | Molecular Weight | 235.098 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 348.6±34.0 °C at 760 mmHg | |
| Molecular Formula | C7H7BrO2S | Melting Point | 103-107 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 164.6±25.7 °C | |
| Name | 1-bromo-4-methylsulfonylbenzene |
|---|---|
| Synonym | More Synonyms |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 348.6±34.0 °C at 760 mmHg |
| Melting Point | 103-107 °C(lit.) |
| Molecular Formula | C7H7BrO2S |
| Molecular Weight | 235.098 |
| Flash Point | 164.6±25.7 °C |
| Exact Mass | 233.934998 |
| PSA | 42.52000 |
| LogP | 1.24 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.564 |
| InChIKey | FJLFSYRGFJDJMQ-UHFFFAOYSA-N |
| SMILES | CS(=O)(=O)c1ccc(Br)cc1 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn,Xi |
| Risk Phrases | R22 |
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2904909090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2904909090 |
|---|---|
| Summary | HS:2904909090 sulphonated, nitrated or nitrosated derivatives of hydrocarbons, whether or not halogenated VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |
|
Cerebrovasodilatation through selective inhibition of the enzyme carbonic anhydrase. 3. 5-(Arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides.
J. Med. Chem. 24(8) , 959-64, (1981) A series of 5-(arylthio)-, 5-(arylsulfinyl)-, and 5-(arylsulfonyl)thiophene-2-sulfonamides is described and anticonvulsant activities are listed for the compounds. In most cases, the sulfones had the ... |
|
|
Efficient green-light-emitting electrochemical cells based on ionic iridium complexes with sulfone-containing cyclometalating ligands.
Chemistry 19(26) , 8597-609, (2013) A new approach to obtain green-emitting iridium(III) complexes is described. The synthetic approach consists of introducing a methylsulfone electron-withdrawing substituent into a 4-phenylpyrazole cyc... |
|
|
Phenoxyphenyl sulfone N-formylhydroxylamines (retrohydroxamates) as potent, selective, orally bioavailable matrix metalloproteinase inhibitors.
J. Med. Chem. 45(1) , 219-32, (2002) A novel series of sulfone N-formylhydroxylamines (retrohydroxamates) have been investigated as matrix metalloproteinases (MMP) inhibitors. The substitution of the ether linkage of ABT-770 (5) with a s... |
| Sulfone, p-bromophenyl methyl (8CI) |
| Sulfone,p-bromophenyl methyl |
| 1-bromo-4-methanesulfonyl-benzene |
| EINECS 222-421-0 |
| Benzene, 1-bromo-4- (methylsulfonyl)- |
| 1-Bromo-4-(methylsulfonyl)benzene |
| 4-MeO2SC6H4Br |
| Sulfone, p-bromophenyl methyl |
| 4-methanesulfonylbromobenzene |
| 4-bromophenyl methyl sulphone |
| Benzene, 1-bromo-4-(methylsulfonyl)- |
| 4-methylsulfonyl-bromobenzene |
| MFCD00025065 |
| p-methyl benzenesulfobromide |
| Methyl 4-bromophenyl sulfone |
| 4-Bromophenyl methyl sulfone |
| p-Bromophenyl methyl sulfone |
 CAS#:104-95-0
CAS#:104-95-0 CAS#:589-87-7
CAS#:589-87-7 CAS#:74-88-4
CAS#:74-88-4 CAS#:60822-47-1
CAS#:60822-47-1 CAS#:934-71-4
CAS#:934-71-4 CAS#:3406-73-3
CAS#:3406-73-3 CAS#:108-86-1
CAS#:108-86-1 CAS#:7143-01-3
CAS#:7143-01-3 CAS#:106-53-6
CAS#:106-53-6 CAS#:105135-96-4
CAS#:105135-96-4 CAS#:5470-49-5
CAS#:5470-49-5 CAS#:340771-31-5
CAS#:340771-31-5 CAS#:21134-15-6
CAS#:21134-15-6![[4-(Methylsulfonyl)phenyl]hydrazine hydrochloride structure](https://image.chemsrc.com/caspic/459/877-66-7.png) CAS#:877-66-7
CAS#:877-66-7 CAS#:701-34-8
CAS#:701-34-8 CAS#:15979-81-4
CAS#:15979-81-4 CAS#:100-25-4
CAS#:100-25-4 CAS#:106-39-8
CAS#:106-39-8
