CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
YS4200000
-
CHEMICAL NAME :
-
Urea, 1-butyl-3-sulfanilyl-
-
CAS REGISTRY NUMBER :
-
339-43-5
-
BEILSTEIN REFERENCE NO. :
-
2218915
-
LAST UPDATED :
-
199707
-
DATA ITEMS CITED :
-
26
-
MOLECULAR FORMULA :
-
C11-H17-N3-O3-S
-
MOLECULAR WEIGHT :
-
271.37
-
WISWESSER LINE NOTATION :
-
ZR DSWMVM4
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
7800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 25,93,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
980 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
DIAEAZ Diabetes. (American Diabetes Assoc., 2 Park Ave., New York, NY 10016) V.1- 1952- Volume(issue)/page/year: 6,2,1957
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2800 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 25,93,1962
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
250 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
NTIS** National Technical Information Service. (Springfield, VA 22161) Formerly U.S. Clearinghouse for Scientific & Technical Information. Volume(issue)/page/year: AD691-490
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
2640 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
REFERENCE :
-
DIAEAZ Diabetes. (American Diabetes Assoc., 2 Park Ave., New York, NY 10016) V.1- 1952- Volume(issue)/page/year: 6,2,1957
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
1920 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - altered sleep time (including change in righting reflex) Behavioral - convulsions or effect on seizure threshold
-
REFERENCE :
-
DIAEAZ Diabetes. (American Diabetes Assoc., 2 Park Ave., New York, NY 10016) V.1- 1952- Volume(issue)/page/year: 6,2,1957 ** OTHER MULTIPLE DOSE TOXICITY DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
307 gm/kg/29W-C
-
TOXIC EFFECTS :
-
Nutritional and Gross Metabolic - other changes Related to Chronic Data - death
-
REFERENCE :
-
DIAEAZ Diabetes. (American Diabetes Assoc., 2 Park Ave., New York, NY 10016) V.1- 1952- Volume(issue)/page/year: 6,2,1957
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
6 gm/kg/24D-I
-
TOXIC EFFECTS :
-
Gastrointestinal - ulceration or bleeding from stomach Endocrine - other changes Related to Chronic Data - death
-
REFERENCE :
-
DIAEAZ Diabetes. (American Diabetes Assoc., 2 Park Ave., New York, NY 10016) V.1- 1952- Volume(issue)/page/year: 6,2,1957
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Mammal - dog
-
DOSE/DURATION :
-
40950 mg/kg/78W-I
-
TOXIC EFFECTS :
-
Blood - hemorrhage Nutritional and Gross Metabolic - dehydration Related to Chronic Data - death
-
REFERENCE :
-
HEPHD2 Handbook of Experimental Pharmacology. (Springer-Verlag, Heidelberger Pl. 3, D-1000 Berlin 33, Fed. Rep. Ger.) V.50- 1978- Volume(issue)/page/year: 119,185,1996
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Primate - monkey
-
DOSE/DURATION :
-
36500 mg/kg/10W-I
-
TOXIC EFFECTS :
-
Lungs, Thorax, or Respiration - chronic pulmonary edema Lungs, Thorax, or Respiration - other changes Related to Chronic Data - death
-
REFERENCE :
-
DIAEAZ Diabetes. (American Diabetes Assoc., 2 Park Ave., New York, NY 10016) V.1- 1952- Volume(issue)/page/year: 6,2,1957 ** REPRODUCTIVE DATA **
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1056 mg/kg
-
SEX/DURATION :
-
female 7-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
PSDTAP Proceedings of the European Society for the Study of Drug Toxicity. (Princeton, NJ 08540) V.1-15, 1963-74. For publisher information, see PESTD5. Volume(issue)/page/year: 11,151,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
REFERENCE :
-
FATOAO Farmakologiya i Toksikologiya (Moscow). For English translation, see PHTXA6 and RPTOAN. (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) V.2- 1939- Volume(issue)/page/year: 28,616,1965
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
5 gm/kg
-
SEX/DURATION :
-
female 9-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - homeostasis
-
REFERENCE :
-
ANENAG Annales d'Endocrinologie. (Masson Editeur, 120 Blvd. Saint-Germain, F-25280 Paris Cedex 06, France) V.1- 1939- Volume(issue)/page/year: 19,167,1958
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
3200 mg/kg
-
SEX/DURATION :
-
female 7-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
REFERENCE :
-
PSDTAP Proceedings of the European Society for the Study of Drug Toxicity. (Princeton, NJ 08540) V.1-15, 1963-74. For publisher information, see PESTD5. Volume(issue)/page/year: 11,151,1970
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
12 gm/kg
-
SEX/DURATION :
-
female 1-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
BANMAC Bulletin de l'Academie Nationale de Medicine (Paris). (Imprimeries Reunies de Chambray, 226, route d'Apremont, F-73490 La Ravoire, France) V.1- 1836- Volume(issue)/page/year: 143,238,1959
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
3 gm/kg
-
SEX/DURATION :
-
female 9 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear
-
REFERENCE :
-
AKGIAO Akushcherstvo i Ginekologiya (Moscow). (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) No.1- 1936- Volume(issue)/page/year: 42(12),35,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
3 gm/kg
-
SEX/DURATION :
-
female 13 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue) Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
AKGIAO Akushcherstvo i Ginekologiya (Moscow). (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) No.1- 1936- Volume(issue)/page/year: 42(12),35,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
1 gm/kg
-
SEX/DURATION :
-
female 1 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
REFERENCE :
-
AKGIAO Akushcherstvo i Ginekologiya (Moscow). (V/O Mezhdunarodnaya Kniga, 113095 Moscow, USSR) No.1- 1936- Volume(issue)/page/year: 42(12),35,1966
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
4400 mg/kg
-
SEX/DURATION :
-
female 5-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
REFERENCE :
-
BSVMA8 Bulletin de la Societe des Sciences Veterinaires et de Medecine Comparee de Lyon. (Lyons, France) V.1-83, 1898-1981. Volume(issue)/page/year: 71,289,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
4400 mg/kg
-
SEX/DURATION :
-
female 5-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - body wall Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
REFERENCE :
-
BSVMA8 Bulletin de la Societe des Sciences Veterinaires et de Medecine Comparee de Lyon. (Lyons, France) V.1-83, 1898-1981. Volume(issue)/page/year: 71,289,1969
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Unreported
-
DOSE :
-
8800 mg/kg
-
SEX/DURATION :
-
female 5-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
REFERENCE :
-
BSVMA8 Bulletin de la Societe des Sciences Veterinaires et de Medecine Comparee de Lyon. (Lyons, France) V.1-83, 1898-1981. Volume(issue)/page/year: 71,289,1969 *** REVIEWS *** TOXICOLOGY REVIEW CLPTAT Clinical Pharmacology and Therapeutics (St. Louis). (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1960- Volume(issue)/page/year: 5,480,1964 TOXICOLOGY REVIEW ARVPAX Annual Review of Pharmacology. (Palo Alto, CA) V.1-15, 1961-75. For publisher information, see ARPTDI. Volume(issue)/page/year: 5,447,1965 TOXICOLOGY REVIEW ADVPA3 Advances in Pharmacology. (New York, NY) V.1-6, 1962-68. For publisher information, see AVPCAQ. Volume(issue)/page/year: 4,263,1966 TOXICOLOGY REVIEW LANCAO Lancet. (7 Adam St., London WC2N 6AD, UK) V.1- 1823- Volume(issue)/page/year: 2,408,1963
|
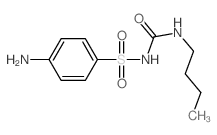





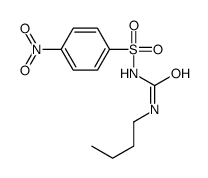

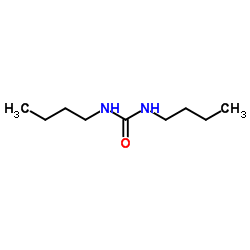
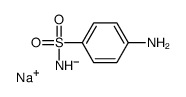
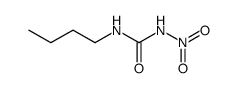
 CAS#:592-31-4
CAS#:592-31-4 CAS#:1773-41-7
CAS#:1773-41-7