Adipostatin A
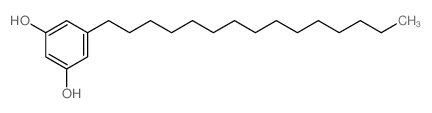
Adipostatin A structure
|
Common Name | Adipostatin A | ||
|---|---|---|---|---|
| CAS Number | 3158-56-3 | Molecular Weight | 320.50900 | |
| Density | 0.96g/cm3 | Boiling Point | 452.5ºC at 760mmHg | |
| Molecular Formula | C21H36O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 195.5ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of Adipostatin A5-Pentadecylresorcinol (Adipostatin A) is a glycerol-3-phosphate dehydrogenase (GPDH) inhibitor with an IC50 of 4.1 µM. Adipostatin A shows good larvicidal activity against Aedes aegypti[1][2]. |
| Name | cardol |
|---|---|
| Synonym | More Synonyms |
| Description | 5-Pentadecylresorcinol (Adipostatin A) is a glycerol-3-phosphate dehydrogenase (GPDH) inhibitor with an IC50 of 4.1 µM. Adipostatin A shows good larvicidal activity against Aedes aegypti[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | 5-Pentadecylresorcinol (Adipostatin A) prevents triglyceride accumulation in 3T3-L1 cells at a concentration of the microM level[1]. |
| References |
| Density | 0.96g/cm3 |
|---|---|
| Boiling Point | 452.5ºC at 760mmHg |
| Molecular Formula | C21H36O2 |
| Molecular Weight | 320.50900 |
| Flash Point | 195.5ºC |
| Exact Mass | 320.27200 |
| PSA | 40.46000 |
| LogP | 6.73150 |
| Index of Refraction | 1.509 |
| InChIKey | KVVSCMOUFCNCGX-UHFFFAOYSA-N |
| SMILES | CCCCCCCCCCCCCCCc1cc(O)cc(O)c1 |
| Storage condition | 2-8°C |
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H317-H318 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 41-43 |
| Safety Phrases | 26-36/37-39 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2907299090 |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2907299090 |
|---|---|
| Summary | 2907299090 polyphenols; phenol-alcohols。supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward)。VAT:17.0%。tax rebate rate:9.0%。MFN tariff:5.5%。general tariff:30.0% |
|
Quantum mechanical and experimental oxidation studies of pentadecylresorcinol, olivetol, orcinol and resorcinol.
Free Radic. Res. 28(4) , 359-68, (1998) Resorcinols (pentadecylresorcinol, olivetol, orcinol and resorcinol) exhibit antioxidant properties in liposomal systems. Antioxidant potency depends on the length of the alkyl chain. Pentadecylresorc... |
|
|
Characterisation of triterpenes and new phenolic lipids in Cameroonian propolis.
Phytochemistry 106 , 156-63, (2014) Chemical investigation of a sample of propolis originating from North-Western Cameroon led to the isolation of thirteen alk(en)ylphenols (1-13) (inseparable mixture) along with α-amyrin (14), β-amyrin... |
|
|
PEG-immobilization of cardol and soluble polymer-supported synthesis of some cardol-coumarin derivatives: preliminary evaluation of their inhibitory activity on mushroom tyrosinase.
Bioorg. Med. Chem. Lett. 19 , 36-9, (2009) In this work, the PEG-immobilization and the liquid phase synthesis of some coumarins derived from cardol are presented. Some preliminary results on their tyrosinase inhibitory activity are also inclu... |
| 1,3-Benzenediol,5-pentadecyl |
| 5-pentadecylbenzene-1,3-diol |
| 1,3-dihydroxy-5-pentadecylbenzene |
| 5-n-pentadecylresorcinol |
| 5-Pentadecyl-1,3-benzenediol |
| Resorcinol,pentadecyl |
| RESORCINOL,5-PENTADECYL |
| Cardol |
| adipostatin A |
| 5-pentadecylresorcinol |
 CAS#:23032-48-6
CAS#:23032-48-6 CAS#:17213-57-9
CAS#:17213-57-9 CAS#:855243-04-8
CAS#:855243-04-8![5-[(8Z)-pentadec-8-enyl]resorcinol Structure](https://image.chemsrc.com/caspic/437/22910-86-7.png) CAS#:22910-86-7
CAS#:22910-86-7 CAS#:129478-09-7
CAS#:129478-09-7 CAS#:88303-25-7
CAS#:88303-25-7 CAS#:629-72-1
CAS#:629-72-1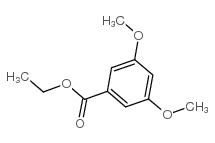 CAS#:17275-82-0
CAS#:17275-82-0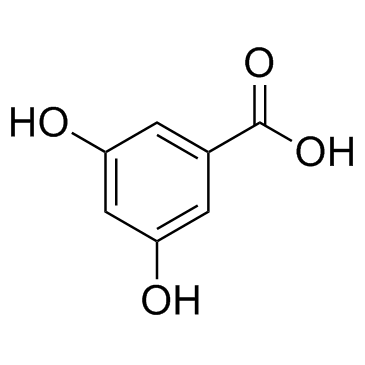 CAS#:99-10-5
CAS#:99-10-5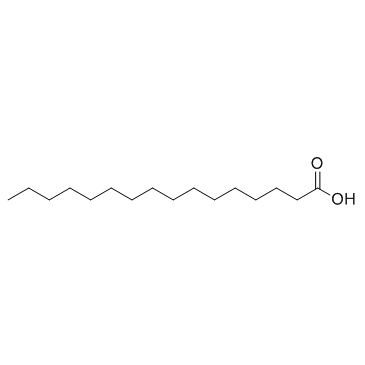 CAS#:57-10-3
CAS#:57-10-3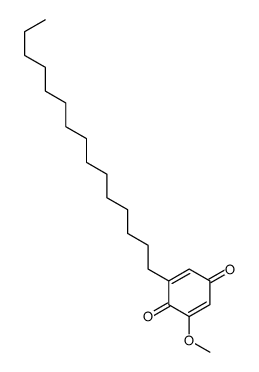 CAS#:144078-11-5
CAS#:144078-11-5