Benzyl 1-piperazinecarboxylate
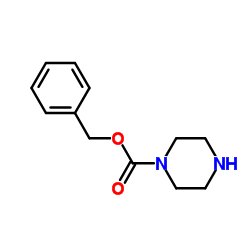
Benzyl 1-piperazinecarboxylate structure
|
Common Name | Benzyl 1-piperazinecarboxylate | ||
|---|---|---|---|---|
| CAS Number | 31166-44-6 | Molecular Weight | 220.27 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 352.4±30.0 °C at 760 mmHg | |
| Molecular Formula | C12H16N2O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 166.9±24.6 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Benzyl 1-piperazinecarboxylateBenzyl piperazine-1-carboxylate is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | benzyl piperazine-1-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Description | Benzyl piperazine-1-carboxylate is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 352.4±30.0 °C at 760 mmHg |
| Molecular Formula | C12H16N2O2 |
| Molecular Weight | 220.27 |
| Flash Point | 166.9±24.6 °C |
| Exact Mass | 220.121185 |
| PSA | 41.57000 |
| LogP | 1.17 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.547 |
| InChIKey | CTOUWUYDDUSBQE-UHFFFAOYSA-N |
| SMILES | O=C(OCc1ccccc1)N1CCNCC1 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933599090 |
| Precursor 5 | |
|---|---|
| DownStream 6 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Synthesis of Macrocyclic, Triazine-Based Receptor Molecules. Löwik DWPM and Lowe CR.
European J. Org. Chem. 2001(15) , 2825-39, (2001)
|
|
|
Novel piperazines: potent melanocortin-4 receptor antagonists with anxiolytic-like activity.
Bioorg. Med. Chem. 15(6) , 2375-85, (2007) In the present study, we found that a novel piperazine compound, 11a, showed a moderate affinity (IC(50)=333nM) for the MC4 receptor. We developed the new type of piperazine compounds and found that m... |
| Benzyl piperazine-1-carboxylate |
| Piperazine-1-carboxylic Acid Benzyl Ester |
| 4-benzyloxycarbonylpiperazine |
| Benzyl 1-piperazinecarboxylate |
| 1-Z-Piperazine |
| N-carbobenzyloxypiperazine |
| 1-(Benzyloxycarbonyl)piperazine |
| 1-Cbz-Piperazine |
| 1-Piperazinecarboxylic acid, phenylmethyl ester |
| benzyl N-piperazinecarboxylate |
| MFCD00274317 |
| phenylmethyl piperazinecarboxylate |
| 1-Carbobenzoxypiperazine |
 CAS#:110-85-0
CAS#:110-85-0 CAS#:501-53-1
CAS#:501-53-1 CAS#:121370-60-3
CAS#:121370-60-3 CAS#:142-63-2
CAS#:142-63-2 CAS#:1885-14-9
CAS#:1885-14-9 CAS#:104740-55-8
CAS#:104740-55-8 CAS#:142-64-3
CAS#:142-64-3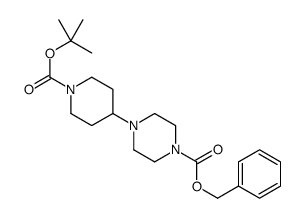 CAS#:177276-40-3
CAS#:177276-40-3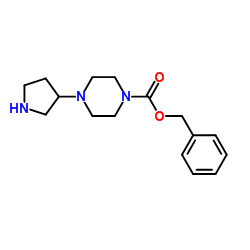 CAS#:436852-08-3
CAS#:436852-08-3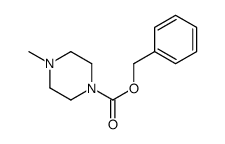 CAS#:46821-51-6
CAS#:46821-51-6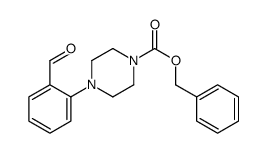 CAS#:626218-86-8
CAS#:626218-86-8
