CH-223191
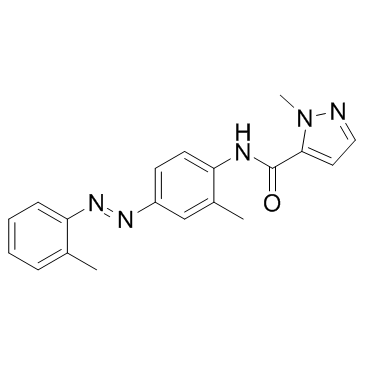
CH-223191 structure
|
Common Name | CH-223191 | ||
|---|---|---|---|---|
| CAS Number | 301326-22-7 | Molecular Weight | 333.387 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 469.4±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H19N5O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 237.7±28.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of CH-223191CH-223191 is a potent and specific antagonist of aryl hydrocarbon receptor (AhR). CH-223191 blocks the binding of TCDD to AhR with an IC50 of 0.03 µM. |
| Name | 2-methyl-N-[2-methyl-4-[(2-methylphenyl)diazenyl]phenyl]pyrazole-3-carboxamide |
|---|---|
| Synonym | More Synonyms |
| Description | CH-223191 is a potent and specific antagonist of aryl hydrocarbon receptor (AhR). CH-223191 blocks the binding of TCDD to AhR with an IC50 of 0.03 µM. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 469.4±45.0 °C at 760 mmHg |
| Molecular Formula | C19H19N5O |
| Molecular Weight | 333.387 |
| Flash Point | 237.7±28.7 °C |
| Exact Mass | 333.158966 |
| PSA | 75.13000 |
| LogP | 3.48 |
| Appearance of Characters | orange-brown |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.633 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: ≥20mg/mL |
|
The aryl hydrocarbon receptor suppresses osteoblast proliferation and differentiation through the activation of the ERK signaling pathway.
Toxicol. Appl. Pharmacol. 280(3) , 502-10, (2014) Ahr activation is known to be associated with synovitis and exacerbated rheumatoid arthritis (RA), but its contributions to bone loss have not been completely elucidated. Osteoblast proliferation and ... |
|
|
In-vitro evidence of enhanced breast cancer resistance protein-mediated intestinal urate secretion by uremic toxins in Caco-2 cells.
J. Pharm. Pharmacol. 67(2) , 170-7, (2015) It has been reported that intestinal urate excretion is increased at chronic kidney disease (CKD) state. In this report, whether uremic toxins are involved in the upregulation of intestinal breast can... |
|
|
Analysis of Glycogen Synthase Kinase Inhibitors That Regulate Cytochrome P450 Expression in Primary Human Hepatocytes by Activation of β-Catenin, Aryl Hydrocarbon Receptor and Pregnane X Receptor Signaling.
Toxicol. Sci. 148 , 261-75, (2015) Cytochrome P450 (CYP) expression and activity are not homogeneous in the liver lobules. Indeed, CYPs are mainly expressed and induced in centrilobular hepatocytes. The wingless-type MMTV integration s... |
| 2-Methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazo-phenyl)-amide |
| 2-methyl-2H-pyrazole-3-carboxylic acid (2-methyl-4-o-tolylazophenyl)amide |
| UNII-HYE7315Z4C |
| 1H-Pyrazole-5-carboxamide, 1-methyl-N-[2-methyl-4-[(E)-2-(2-methylphenyl)diazenyl]phenyl]- |
| 1-Methyl-N-{2-methyl-4-[(E)-(2-methylphenyl)diazenyl]phenyl}-1H-pyrazole-5-carboxamide |
| AhR Antagonist |
| CH-223191 |