Bavachalcone
Modify Date: 2025-08-20 17:43:54
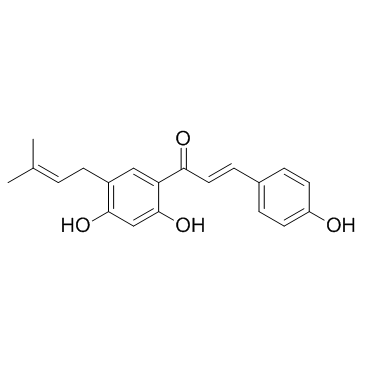
Bavachalcone structure
|
Common Name | Bavachalcone | ||
|---|---|---|---|---|
| CAS Number | 28448-85-3 | Molecular Weight | 324.370 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 549.6±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H20O4 | Melting Point | 168-169℃ | |
| MSDS | N/A | Flash Point | 300.2±26.6 °C | |
Use of BavachalconeBavachalcone is a major bioactive compounds isolated from Psoralea corylifolia L.; has been widely used as traditional Chinese medicine; antibiotic or anticancer agent.IC50 value:Target:Bavachalcone inhibited osteoclast formation from precursor cells with the IC(50) of approximately 1.5 microg ml(-1). The activation of MEK, ERK, and Akt by receptor activator of nuclear factor kappaB ligand (RANKL), the osteoclast differentiation factor, was prominently reduced in the presence of bavachalcone. The induction of c-Fos and NFATc1, key transcription factors for osteoclastogenesis, by RANKL was also suppressed by bavachalcone [1]. Bavachalcone exhibited a significant inhibitory effect on baculovirus-expressed BACE-1 in vitro [2]. Bavachalcone had stronger inhibition on UGT1A1 and UGT1A7 than corylin which did not inhibit UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A10, and UGT2B4. Data fitting using Dixon and Lineweaver-Burk plots demonstrated the noncompetitive inhibition of bavachalcone against UGT1A1 and UGT1A7-mediated 4-MU glucuronidation reaction. The values of inhibition kinetic parameters (Ki) were 5.41 μ M and 4.51μ M for UGT1A1 and UGT1A7, respectively [3]. |
| Name | (E)-1-[2,4-dihydroxy-5-(3-methylbut-2-enyl)phenyl]-3-(4-hydroxyphenyl)prop-2-en-1-one |
|---|---|
| Synonym | More Synonyms |
| Description | Bavachalcone is a major bioactive compounds isolated from Psoralea corylifolia L.; has been widely used as traditional Chinese medicine; antibiotic or anticancer agent.IC50 value:Target:Bavachalcone inhibited osteoclast formation from precursor cells with the IC(50) of approximately 1.5 microg ml(-1). The activation of MEK, ERK, and Akt by receptor activator of nuclear factor kappaB ligand (RANKL), the osteoclast differentiation factor, was prominently reduced in the presence of bavachalcone. The induction of c-Fos and NFATc1, key transcription factors for osteoclastogenesis, by RANKL was also suppressed by bavachalcone [1]. Bavachalcone exhibited a significant inhibitory effect on baculovirus-expressed BACE-1 in vitro [2]. Bavachalcone had stronger inhibition on UGT1A1 and UGT1A7 than corylin which did not inhibit UGT1A1, UGT1A3, UGT1A7, UGT1A8, UGT1A10, and UGT2B4. Data fitting using Dixon and Lineweaver-Burk plots demonstrated the noncompetitive inhibition of bavachalcone against UGT1A1 and UGT1A7-mediated 4-MU glucuronidation reaction. The values of inhibition kinetic parameters (Ki) were 5.41 μ M and 4.51μ M for UGT1A1 and UGT1A7, respectively [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 549.6±50.0 °C at 760 mmHg |
| Melting Point | 168-169℃ |
| Molecular Formula | C20H20O4 |
| Molecular Weight | 324.370 |
| Flash Point | 300.2±26.6 °C |
| Exact Mass | 324.136169 |
| PSA | 77.76000 |
| LogP | 5.21 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.658 |
| Storage condition | -20°C |
| (2E)-1-[2,4-Dihydroxy-5-(3-methyl-2-buten-1-yl)phenyl]-3-(4-hydroxyphenyl)-2-propen-1-one |
| Bavachalcone |
| (E)-1-(2,4-Dihydroxy-5-(3-methyl-but-2-enyl)-phenyl)-3-(4-hydroxy-phenyl)-propenone |
| 2-Propen-1-one, 1-[2,4-dihydroxy-5-(3-methyl-2-buten-1-yl)phenyl]-3-(4-hydroxyphenyl)-, (2E)- |
| buroussochalcone B |
| broussochalcone B |
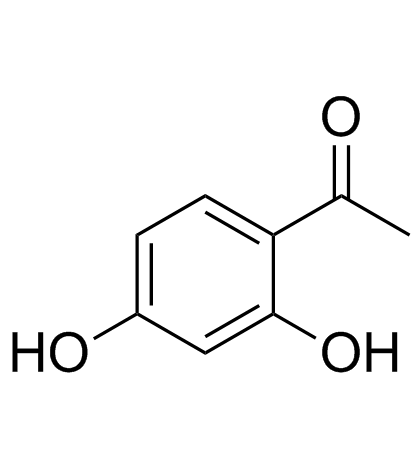 CAS#:89-84-9
CAS#:89-84-9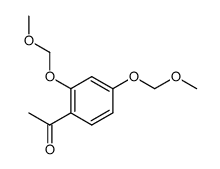 CAS#:6515-05-5
CAS#:6515-05-5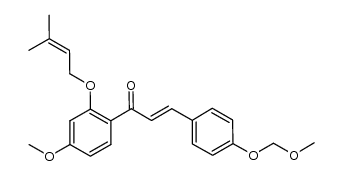 CAS#:1092462-95-7
CAS#:1092462-95-7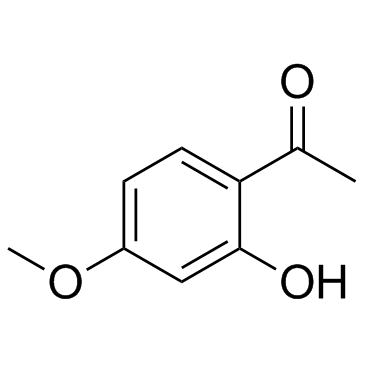 CAS#:552-41-0
CAS#:552-41-0 CAS#:6515-21-5
CAS#:6515-21-5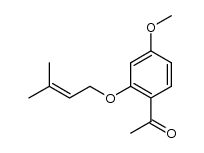 CAS#:128961-25-1
CAS#:128961-25-1
