Methyl cyclopentene-1-carboxylate
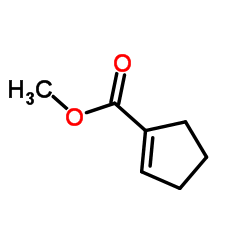
Methyl cyclopentene-1-carboxylate structure
|
Common Name | Methyl cyclopentene-1-carboxylate | ||
|---|---|---|---|---|
| CAS Number | 25662-28-6 | Molecular Weight | 126.153 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 161.3±19.0 °C at 760 mmHg | |
| Molecular Formula | C7H10O2 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 50.5±18.9 °C | |
| Name | methyl cyclopentene-1-carboxylate |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 161.3±19.0 °C at 760 mmHg |
| Molecular Formula | C7H10O2 |
| Molecular Weight | 126.153 |
| Flash Point | 50.5±18.9 °C |
| Exact Mass | 126.068077 |
| PSA | 26.30000 |
| LogP | 1.88 |
| Vapour Pressure | 2.3±0.3 mmHg at 25°C |
| Index of Refraction | 1.483 |
| InChIKey | VTYCAXIAUKEGBQ-UHFFFAOYSA-N |
| SMILES | COC(=O)C1=CCCC1 |
| Personal Protective Equipment | Eyeshields;Gloves;half-mask respirator (US);multi-purpose combination respirator cartridge (US) |
|---|---|
| Hazard Codes | Xi |
| Safety Phrases | S23-S24/25 |
| WGK Germany | 3 |
| HS Code | 2916209090 |
| HS Code | 2916209090 |
|---|---|
| Summary | 2916209090 other cyclanic, cyclenic or cyclotherpenic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward) MFN tariff:6.5% General tariff:30.0% |
|
Asymmetric intermolecular boron Heck-type reactions via oxidative palladium(II) catalysis with chiral tridentate NHC-amidate-alkoxide ligands.
J. Org. Chem. 75(1) , 95-101, (2010) Chiral dimeric tridentate NHC-amidate-alkoxide palladium(II) complexes, 3a and 3b, effected oxidative boron Heck-type reactions of aryl boronic acids with both acyclic and cyclic alkenes at room tempe... |
|
|
Formal syntheses of (+/-)-pinnaic acid and (+/-)-halichlorine.
Org. Lett. 7(25) , 5733-5, (2005) [chemical reaction: see text]. Concise formal syntheses of marine alkaloids (+/-)-pinnaic acid (1) and (+/-)-halichlorine (2) have been accomplished from a common intermediate. The syntheses illustrat... |
|
|
THE ORGANIC CHEMISTRY NOTEBOOK SERIES, A DIDACTICAL APPROACH,®(I) A THEORETICAL MECHANISTIC APPROACH TO DIASTEROSELECTIVE SYNTHESIS OF CIS-1, 2-DIALKENYLCYCLOPROPANOLS AND SUBSEQUENT OXY-COPE REARRANGEMENT BY JIN KUN CHA ET AL. Bravo J.
Rev. Boliv. Quim. 22(1) , 1-10, (2005)
|
| 1-cyclopenatnecarboxylic acid methyl ester |
| MFCD00239506 |
| 1-Cyclopentene-1-carboxylic acid, methyl ester |
| L5UTJ AVO1 |
| Cyclopenten-1-carbonsaeure-methyl-ester |
| Cyclopentene-1-carboxylic acid methyl ester |
| Methyl 1-cyclopentene-1-carboxylate |
| methyl 1-cyclopenten-1-carboxylate |
| methyl cyclopent-1-enecarboxylate |
| cyclopent-1-enecarboxylic acid methyl ester |
| Methyl cyclopentene-1-carboxylate |
| Methyl cyclopent-1-ene-1-carboxylate |
| methyl-1-cyclopentene-1-carboxylate |

