ABT-510
Modify Date: 2025-08-26 14:52:32
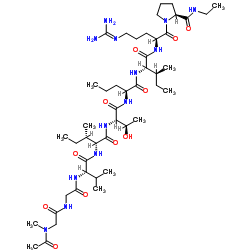
ABT-510 structure
|
Common Name | ABT-510 | ||
|---|---|---|---|---|
| CAS Number | 251579-55-2 | Molecular Weight | 994.23200 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C46H83N13O11 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of ABT-510ABT-510 is an anti-angiogenic TSP peptide (Thrombospondin-1 analogue) that induces apoptosis and inhibits ovarian tumour growth in an orthotopic, syngeneic model of epithelial ovarian cancer. ABT-510 also reduces angiogenesis and inflammatory responses in a murine model of inflammatory bowel disease. ABT-510 can be used in studies of cancer (particularly epithelial ovarian cancer) and inflammatory bowel disease (IBD)[1][2]. |
| Name | N-Acetyl-N-methylglycylglycyl-L-valyl-D-alloisoleucyl-L-threonyl-L-norvalyl-L-isoleucyl-N5-(diaminomethylene)-L-ornithyl-N-ethyl-L-prolinamide |
|---|---|
| Synonym | More Synonyms |
| Description | ABT-510 is an anti-angiogenic TSP peptide (Thrombospondin-1 analogue) that induces apoptosis and inhibits ovarian tumour growth in an orthotopic, syngeneic model of epithelial ovarian cancer. ABT-510 also reduces angiogenesis and inflammatory responses in a murine model of inflammatory bowel disease. ABT-510 can be used in studies of cancer (particularly epithelial ovarian cancer) and inflammatory bowel disease (IBD)[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | ABT-510 (1, 5, 10, 20, 50 nM; 24 h) induces apoptosis in ID8 cells and (50 nM; 24 h) increases the incidence of apoptosis in the human epithelial cancer cell lines SKOV3, OVCAR3, and CAOV3[1]. Apoptosis Analysis[1] Cell Line: ID8, SKOV3, OVCAR3, and CAOV3 cells Concentration: 1, 5, 10, 20, 50 nM Incubation Time: 24 h Result: Induced ID8 cells apoptosis and increased in apoptosis in the human EOC cell lines SKOV3, OVCAR3, and CAOV3. |
| In Vivo | ABT-510 (100 mg/kg; i.p.; single daily for 90 days) leads to a significant reduction in tumor size, ascites fluid volume, and secondary lesion dissemination in mice[1]. ABT-510 (100 mg/kg; I.p.; single daily for 90 days) induces cells apoptosis in vivo[1]. ABT-510 (60 mg/kg; osmotic minipumps for s.c.; single daily for 7 days) decreases angiogenesis and inflammation in a murine model of inflammatory bowel disease[2]. Animal Model: TSP-1-Null mice (C57BL/6 background; orthotopic, syngeneic model of epithelial ovarian cancer)[1]. Dosage: 100 mg/kg Administration: Intraperitoneal injection; single daily for 90 days Result: Reduced ovarian tumor growth in wild-type and TSP-1-Null Mice. Significantly reduced the volume of ascites and completely abolished the formation of peritoneal lesions. Reversed ovarian tumor hypervascularization and increased the proportion of mature blood vessels. Animal Model: TSP-1-Null mice (C57BL/6 background; 6-week-old; DSS-induced inflammatory bowel disease murine model)[2]. Dosage: 60 mg/kg Administration: Subcutaneously implanted osmotic minipumps (0.5µL/h); single daily for 7 days Result: Significantly delayed DSS-induced bleeding and improved the overall severity of disease.Significantly diminished inflammation grading and angiogenesis |
| Molecular Formula | C46H83N13O11 |
|---|---|
| Molecular Weight | 994.23200 |
| Exact Mass | 993.63400 |
| PSA | 358.05000 |
| LogP | 2.06520 |
| L-Prolinamide, N-acetyl-N-methylglycylglycyl-L-valyl-D-alloisoleucyl-L-threonyl-L-norvalyl-L-isoleucyl-N-(diaminomethylene)-L-ornithyl-N-ethyl- |
| N-Acetyl-N-methylglycylglycyl-L-valyl-D-alloisoleucyl-L-threonyl-L-norvalyl-L-isoleucyl-N-(diaminomethylene)-L-ornithyl-N-ethyl-L-prolinamide |