Dexamethasone-4,6α,21,21-d4
Modify Date: 2024-01-11 17:51:10
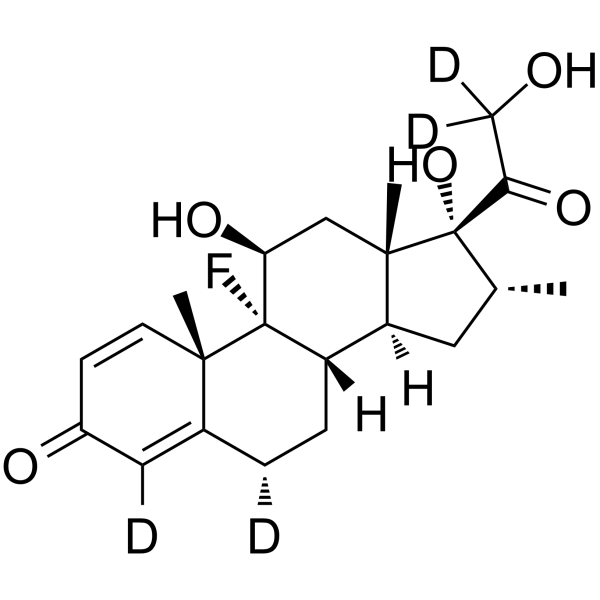
Dexamethasone-4,6α,21,21-d4 structure
|
Common Name | Dexamethasone-4,6α,21,21-d4 | ||
|---|---|---|---|---|
| CAS Number | 2483831-63-4 | Molecular Weight | 396.49 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H25D4FO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Dexamethasone-4,6α,21,21-d4Dexamethasone-4,6α,21,21-d4 is the deuterium labeled Dexamethasone-4,6α,21,21. Dexamethasone (Hexadecadrol) is a glucocorticoid receptor agonist. Dexamethasone also significantly decreases CD11b, CD18, and CD62L expression on neutrophils, and CD11b and CD18 expression on monocytes. Dexamethasone is highly effective in the control of COVID-19 infection. Dexamethasone inhibits production of exosomes containing inflammatory microRNA-155 in lipopolysaccharide-induced macrophage inflammatory responses. |
| Name | Dexamethasone-4,6α,21,21-d4 |
|---|
| Description | Dexamethasone-4,6α,21,21-d4 is the deuterium labeled Dexamethasone-4,6α,21,21. Dexamethasone (Hexadecadrol) is a glucocorticoid receptor agonist. Dexamethasone also significantly decreases CD11b, CD18, and CD62L expression on neutrophils, and CD11b and CD18 expression on monocytes. Dexamethasone is highly effective in the control of COVID-19 infection. Dexamethasone inhibits production of exosomes containing inflammatory microRNA-155 in lipopolysaccharide-induced macrophage inflammatory responses. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C22H25D4FO5 |
|---|---|
| Molecular Weight | 396.49 |