Triglycidyl isocyanurate
Modify Date: 2024-01-01 21:56:06
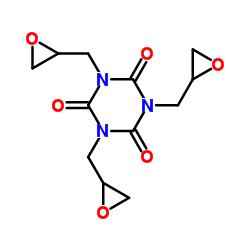
Triglycidyl isocyanurate structure
|
Common Name | Triglycidyl isocyanurate | ||
|---|---|---|---|---|
| CAS Number | 2451-62-9 | Molecular Weight | 297.264 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 501.1±15.0 °C at 760 mmHg | |
| Molecular Formula | C12H15N3O6 | Melting Point | 95-98°C | |
| MSDS | Chinese USA | Flash Point | 256.9±20.4 °C | |
| Symbol |
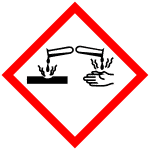


GHS05, GHS06, GHS08 |
Signal Word | Danger | |
Use of Triglycidyl isocyanurateTriglycidyl isocyanurate (TGIC; Teroxirone) is a triazene triepoxide with antiangiogenic and antineoplastic activities. Triglycidyl isocyanurate inhibits the growth of non-small-cell-lung cancer cells via p53 activation. Triglycidyl isocyanurate induces cell apoptosis. Triglycidyl isocyanurate can be used for cancer research[1][2]. |
| Name | 1,3,5-Triglycidyl isocyanurate |
|---|---|
| Synonym | More Synonyms |
| Description | Triglycidyl isocyanurate (TGIC; Teroxirone) is a triazene triepoxide with antiangiogenic and antineoplastic activities. Triglycidyl isocyanurate inhibits the growth of non-small-cell-lung cancer cells via p53 activation. Triglycidyl isocyanurate induces cell apoptosis. Triglycidyl isocyanurate can be used for cancer research[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Triglycidyl isocyanurate (0-30 μM; 48 hours) reduces the growth of spheroids of human non-small-cell-lung cancer cells in culture, it leads to a gradual reduction in size for tumorspheres of A549, H460 and H1299 cells[1]. Triglycidyl isocyanurate (0-30 μM; 48 hours) inhibits expression of akt1/2/3 and phosphorylated Aktser473/474/472 of A549, H460 and H1299 tumorspheres, however, the cleavage of PARP and procaspase-3 plus the emergent active caspase-3 fragment are only visible in H460 and A549 tumorspheres[1]. Cell Viability Assay[1] Cell Line: A549, H460 and H1299 cells Concentration: 0 μM; 5 μM; 10 μM; 30 μM Incubation Time: 48 hours Result: Inhibited tumor cells growth in soft agar. Western Blot Analysis[1] Cell Line: A549, H460 and H1299 cells Concentration: 0 μM; 5 μM; 10 μM; 30 μM Incubation Time: 48 hours Result: Inhibited akt1/2/3 expression and p-aktser473/474/472 expression of A549, H460 and H1299 tumorspheres |
| In Vivo | Triglycidyl isocyanurate (subcutaneous injection; 1.8 and 3.6 mg/kg; every 2–3 days for total seven times; 30 days) suppresses the growth of xenograft tumors and has no effects on weight in nude mice[2]. Animal Model: Female nu/nu mice with Huh7 cells subcutaneously injected into the dorsal area[2] Dosage: 1.8 mg/kg and 3.6 mg/kg Administration: Subcutaneous injection; every 2–3 days for total seven times; 30 days Result: Inhibited the growth of xenograft tumors. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 501.1±15.0 °C at 760 mmHg |
| Melting Point | 95-98°C |
| Molecular Formula | C12H15N3O6 |
| Molecular Weight | 297.264 |
| Flash Point | 256.9±20.4 °C |
| Exact Mass | 297.096100 |
| PSA | 103.59000 |
| LogP | -2.77 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.635 |
| Water Solubility | <0.1 g/100 mL at 20 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS05, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H331-H317-H318-H340-H373-H412 |
| Precautionary Statements | P201-P261-P273-P280-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic |
| Risk Phrases | R23/25;R41;R43;R48/22;R52/53 |
| Safety Phrases | S53-S45-S61 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | XZ1994900 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933699090 |
| Precursor 0 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933699090 |
|---|---|
| Summary | 2933699090 other compounds containing an unfused triazine ring (whether or not hydrogenated) in the structure。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:20.0% |
|
Determination of triglycidyl isocyanurate from air samples by ultra-performance liquid chromatography coupled with coordination ion spray mass spectrometry.
Rapid Commun. Mass Spectrom. 29 , 913-8, (2015) Ultra-performance liquid chromatography (UPLC) coupled with coordination ion spray tandem mass spectrometry was used for the analysis of air samples containing triglycidyl isocyanurate. The method is ... |
|
|
Pharmacological characterization of teroxirone, a triepoxide antitumor agent, in rats, rabbits, and humans.
Cancer Res. 44(9) , 4151-6, (1984) Teroxirone is an experimental triepoxide antitumor agent currently undergoing evaluation in clinical trials. We have developed an assay based on derivatization with diethyldithiocarbamate followed by ... |
|
|
Occupational contact dermatitis from triglycidyl isocyanurate in a powder paint factory.
Contact Dermatitis 26(1) , 59, (1992)
|
| XB 2615 |
| tgt |
| (RS,RS,SR)-1,3,5-Tris(2,3-epoxypropyl)-s-triazine-2,4,6(1H,3H,5H)-trione |
| Teroxirone |
| EINECS 219-514-3 |
| TGIC |
| 1,3,5-Tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione,TGIC,Triglycidyl isocyanurate |
| 1,3,5-Triazine-2,4,6(1H,3H,5H)-trione, 1,3,5-tris(oxiranylmethyl)- |
| 1,3,5-Tris(2-oxiranylmethyl)-1,3,5-triazinane-2,4,6-trione |
| Isocyanuric Acid Triglycidyl Ester |
| T6NVNVNVJ A1- BT3OTJ& C1- BT3OTJ& E1- BT3OTJ |
| 1,3,5-triglycidyl-s-triazinetrione |
| MFCD00080670 |
| araldite pt-810 |
| triglycidyl |
| Triglycidyl Isocyanurate |
| TEPIC |
| Tris(2,3-epoxypropyl) Isocyanurate |
| TGIC,triglycidyl isocyanurate |
| 1,3,5-Tris(oxiran-2-ylmethyl)-1,3,5-triazinane-2,4,6-trione |
| glycidylisocyanurate |
| Isocyanuric Acid Tris(2,3-epoxypropyl) Ester |
 CAS#:101-05-3
CAS#:101-05-3 CAS#:1665-48-1
CAS#:1665-48-1
