2-Amino-3,5-dibromopyrazine
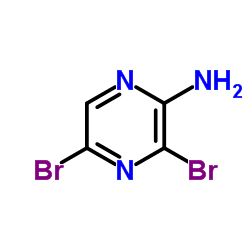
2-Amino-3,5-dibromopyrazine structure
|
Common Name | 2-Amino-3,5-dibromopyrazine | ||
|---|---|---|---|---|
| CAS Number | 24241-18-7 | Molecular Weight | 252.89 | |
| Density | 2.3±0.1 g/cm3 | Boiling Point | 294.6±35.0 °C at 760 mmHg | |
| Molecular Formula | C4H3Br2N3 | Melting Point | 114-117 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 131.9±25.9 °C | |
| Symbol |


GHS05, GHS06 |
Signal Word | Danger | |
Use of 2-Amino-3,5-dibromopyrazineAmino-3,5-dibromopyrazine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 3,5-dibromopyrazin-2-amine |
|---|---|
| Synonym | More Synonyms |
| Description | Amino-3,5-dibromopyrazine is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 2.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 294.6±35.0 °C at 760 mmHg |
| Melting Point | 114-117 °C(lit.) |
| Molecular Formula | C4H3Br2N3 |
| Molecular Weight | 252.89 |
| Flash Point | 131.9±25.9 °C |
| Exact Mass | 250.869354 |
| PSA | 51.80000 |
| LogP | 3.05 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.686 |
| InChIKey | DTLBKXRFWUERQN-UHFFFAOYSA-N |
| SMILES | Nc1ncc(Br)nc1Br |
| Storage condition | Refrigerator |
| Symbol |


GHS05, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H315-H318-H335 |
| Precautionary Statements | P261-P280-P301 + P310-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22;R37/38;R41 |
| Safety Phrases | S26-S36/39 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1 |
| HS Code | 2933990090 |
|
~93% 
2-Amino-3,5-dib... CAS#:24241-18-7 |
| Literature: Paudler, William W.; Jovanovic, Misa V. Journal of Organic Chemistry, 1983 , vol. 48, # 7 p. 1064 - 1069 |
|
~12% 
2-Amino-3,5-dib... CAS#:24241-18-7 |
| Literature: Chemical Communications, , vol. 47, # 16 p. 4688 - 4690 |
|
~6% 
2-Amino-3,5-dib... CAS#:24241-18-7 |
| Literature: Journal of Heterocyclic Chemistry, , vol. 19, # 3 p. 673 - 674 |
|
~% 
2-Amino-3,5-dib... CAS#:24241-18-7 |
| Literature: Heterocycles, , vol. 86, # 2 p. 1323 - 1339 |
| Precursor 2 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Studies on pyrazines. XX, A simple synthesis of 5-substituted 2-amino-3-cyanopyrazines: useful intermediates for pteridine synthesis. Synthesis 8 (1990): 659-660. Sato N and Takeuchi R. Synthesis 8 , 659-660, (1990)
|
|
|
Synthesis of N-Substituted-2-Aminothiazolo [4,5-b] pyrazines by Tandem Reaction of o-Aminohalopyrazines with Isothiocyanates. Kwak SH, et al.
Bull. Korean Chem. Soc. 33 , 4271-4274, (2012)
|
|
|
Synthesis of Tetraaza Derivatives of Benzoxazinophenothiazine. Ezema BE, et al.
Orient. J. Chem. 31(1) , 133-139, (2015)
|
| 2-Pyrazinamine, 3,5-dibromo- |
| 3-amino-2,6-dibromopyrazine |
| MFCD00673150 |
| 3,5-Dibromo-2-pyrazinamine |
| 2-Amino-3,5-dibromo-1,4-diazine |
| 3,5-Dibromopyrazine-2-ylamine |
| 5-dibromo-2-aminopyrazine |
| 3,5-dibromo-pyrazin-2-ylamine |
| 2-Amino-3,5-dibromopyrazine |
| 3,5-Dibrom-2-pyrazinamin |

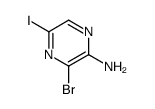
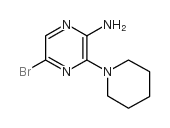 CAS#:90674-84-3
CAS#:90674-84-3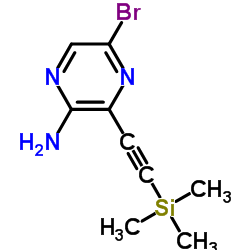 CAS#:875781-41-2
CAS#:875781-41-2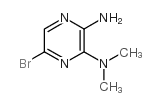 CAS#:89641-34-9
CAS#:89641-34-9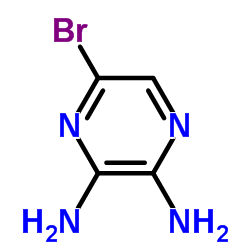 CAS#:89123-58-0
CAS#:89123-58-0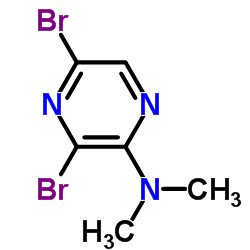 CAS#:84539-07-1
CAS#:84539-07-1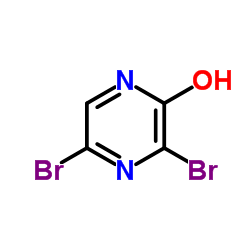 CAS#:21943-15-7
CAS#:21943-15-7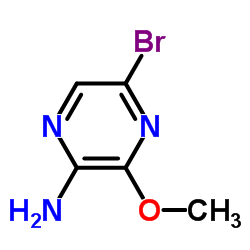 CAS#:5900-13-0
CAS#:5900-13-0 CAS#:77112-66-4
CAS#:77112-66-4
