2-naphthoic chloride

2-naphthoic chloride structure
|
Common Name | 2-naphthoic chloride | ||
|---|---|---|---|---|
| CAS Number | 2243-83-6 | Molecular Weight | 190.626 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 307.4±11.0 °C at 760 mmHg | |
| Molecular Formula | C11H7ClO | Melting Point | 50-52 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 141.2±12.4 °C | |
| Symbol |

GHS05 |
Signal Word | Danger | |
| Name | naphthalene-2-carbonyl chloride |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 307.4±11.0 °C at 760 mmHg |
| Melting Point | 50-52 °C(lit.) |
| Molecular Formula | C11H7ClO |
| Molecular Weight | 190.626 |
| Flash Point | 141.2±12.4 °C |
| Exact Mass | 190.018539 |
| PSA | 17.07000 |
| LogP | 3.44 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.645 |
| Storage condition | 2-8°C |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H314 |
| Precautionary Statements | P280-P305 + P351 + P338-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | C:Corrosive; |
| Risk Phrases | R34 |
| Safety Phrases | S26-S36/37/39-S45 |
| RIDADR | UN 3261 8/PG 2 |
| WGK Germany | 3 |
| Packaging Group | II |
| Hazard Class | 8 |
| HS Code | 29163900 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2916399090 |
|---|---|
| Summary | 2916399090 other aromatic monocarboxylic acids, their anhydrides, halides, peroxides, peroxyacids and their derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Liquid chromatographic chiral stationary phases in pharmaceutical analysis: determination of trace amounts of the (-)-enantiomer in (+)-amphetamine.
J. Pharm. Sci. 73(8) , 1162-4, (1984) A rapid and accurate method was developed for the determination of the enantiomeric composition of amphetamine preparations. Amide derivatives of the amphetamine enantiomers are first formed by using ... |
|
|
High-performance liquid chromatographic determination of mexiletine enantiomers in plasma using direct and indirect enantioselective separations.
J. Chromatogr. B, Biomed. Appl. 685(2) , 281-9, (1996) Two methods were developed for the determination of mexiletine enantiomers in plasma samples suitable for studies on the stereoselective disposition of this drug. Both methods used fluorescence detect... |
|
|
A chemically modified graphite electrode for electrocatalytic oxidation of reduced nicotinamide adenine dinucleotide based on a phenothiazine derivative, 3-ß-naphthoyl-toluidine blue O. Persson B.
J. Electroanal. Chem. Interfac. Electrochem. 287(1) , 61-80, (1990)
|
| 2-NAPHTHALENECARBONYL CHLORIDE |
| Naphthalen-2-carbonylchlorid |
| 2-naphthoyl-Cl |
| EINECS 218-822-5 |
| MFCD00004093 |
| 2-(Chlorocarbonyl)naphthalene |
| 2-Naphthoyl chloride |
| 2-Napthoyl chloride |
| 2-naphthoic chloride |
| β-Naphthalenecarbonyl chloride |
| 1,2,3,4-tetrahydro-2-naphthoyl chloride |
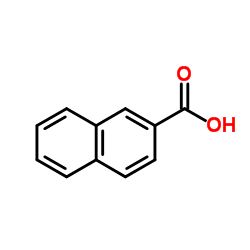 CAS#:93-09-4
CAS#:93-09-4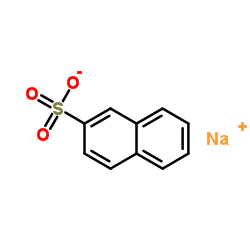 CAS#:532-02-5
CAS#:532-02-5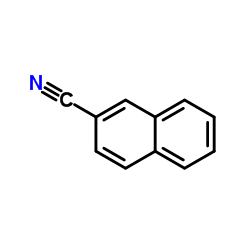 CAS#:613-46-7
CAS#:613-46-7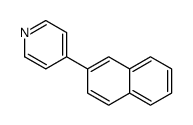 CAS#:111876-50-7
CAS#:111876-50-7 CAS#:105855-20-7
CAS#:105855-20-7 CAS#:107574-57-2
CAS#:107574-57-2 CAS#:110876-52-3
CAS#:110876-52-3 CAS#:39627-84-4
CAS#:39627-84-4 CAS#:362669-46-3
CAS#:362669-46-3 CAS#:362669-52-1
CAS#:362669-52-1![2-[(trichloromethyl)thio]pyridine structure](https://image.chemsrc.com/caspic/315/66832-24-4.png) CAS#:66832-24-4
CAS#:66832-24-4 CAS#:580-13-2
CAS#:580-13-2 CAS#:14625-56-0
CAS#:14625-56-0
