Loganic acid
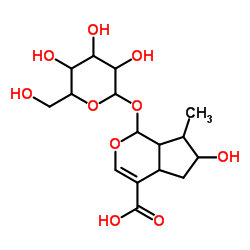
Loganic acid structure
|
Common Name | Loganic acid | ||
|---|---|---|---|---|
| CAS Number | 22255-40-9 | Molecular Weight | 376.356 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | 646.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C16H24O10 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 235.6±25.0 °C | |
Use of Loganic acidLoganic acid is an iridoid isolated from cornelian cherry fruits. Loganic acid can modulate diet-induced atherosclerosis and redox status. Loganic acid has strong free radical scavenging activity and remarkable cyto-protective effect against heavy metal mediated toxicity[1][2]. |
| Name | loganic acid |
|---|---|
| Synonym | More Synonyms |
| Description | Loganic acid is an iridoid isolated from cornelian cherry fruits. Loganic acid can modulate diet-induced atherosclerosis and redox status. Loganic acid has strong free radical scavenging activity and remarkable cyto-protective effect against heavy metal mediated toxicity[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 646.3±55.0 °C at 760 mmHg |
| Molecular Formula | C16H24O10 |
| Molecular Weight | 376.356 |
| Flash Point | 235.6±25.0 °C |
| Exact Mass | 376.136932 |
| PSA | 166.14000 |
| LogP | -2.49 |
| Vapour Pressure | 0.0±4.4 mmHg at 25°C |
| Index of Refraction | 1.634 |
| Storage condition | ?20°C |
| RIDADR | NONH for all modes of transport |
|---|
|
Analysis of iridoids, secoiridoids and xanthones in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea using LC-MS and RP-HPLC.
J. Pharm. Biomed. Anal. 54(3) , 517-25, (2011) This study presents a new and validated HPLC method for the simultaneous determination of bioactive compounds in Centaurium erythraea, Frasera caroliniensis and Gentiana lutea. The iridoid loganic aci... |
|
|
A novel lactone from Tripterospermum chinense.
Yao Xue Xue Bao 47(11) , 1517-20, (2012) A novel lactone, tripterospermumcins E (1), along with four known compounds, sweroside (2), loganic acid (3), 8-epi-kingiside (4) and bergenin (5), were isolated from the aerial parts of Tripterosperm... |
|
|
The leaf epidermome of Catharanthus roseus reveals its biochemical specialization.
Plant Cell 20(3) , 524-42, (2008) Catharanthus roseus is the sole commercial source of the monoterpenoid indole alkaloids (MIAs), vindoline and catharanthine, components of the commercially important anticancer dimers, vinblastine and... |
| 1-(Hexopyranosyloxy)-6-hydroxy-7-methyl-1,4a,5,6,7,7a-hexahydrocyclopenta[c]pyran-4-carboxylic acid |
| 6-Hydroxy-7-methyl-1-(3,4,5-trihydroxy-6-hydroxymethyl-tetrahydro-pyran-2-yloxy)-1,4a,5,6,7,7a-hexahydro-cyclopenta[c]pyran-4-carboxylic acid |
| Loganic acid |
| Norloganin |
| Cyclopenta[c]pyran-4-carboxylic acid, 1-(hexopyranosyloxy)-1,4a,5,6,7,7a-hexahydro-6-hydroxy-7-methyl- |

