1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine
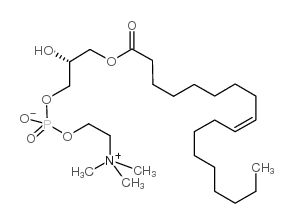
1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine structure
|
Common Name | 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine | ||
|---|---|---|---|---|
| CAS Number | 19420-56-5 | Molecular Weight | 521.66700 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C26H52NO7P | Melting Point | N/A | |
| MSDS | USA | Flash Point | N/A | |
| Name | 1-Oleoyl-sn-glycero-3-phosphocholine |
|---|---|
| Synonym | More Synonyms |
| Molecular Formula | C26H52NO7P |
|---|---|
| Molecular Weight | 521.66700 |
| Exact Mass | 521.34800 |
| PSA | 114.93000 |
| LogP | 6.20600 |
| InChIKey | YAMUFBLWGFFICM-PTGWMXDISA-N |
| SMILES | CCCCCCCCC=CCCCCCCCC(=O)OCC(O)COP(=O)([O-])OCC[N+](C)(C)C |
| Storage condition | -20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 22-24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2923900090 |
|
~% 
1-oleoyl-2-hydr... CAS#:19420-56-5 |
| Literature: Chem SPA Patent: US2006/79703 A1, 2006 ; Location in patent: Page/Page column 3-4 ; |
|
~97% 
1-oleoyl-2-hydr... CAS#:19420-56-5 |
| Literature: Fasoli, Ezio; Arnone, Alberto; Caligiuri, Antonio; D'Arrigo, Paola; De Ferra, Lorenzo; Servi, Stefano Organic and Biomolecular Chemistry, 2006 , vol. 4, # 15 p. 2974 - 2978 |
|
~% 
1-oleoyl-2-hydr... CAS#:19420-56-5 |
| Literature: CHEMI S.p.A. Patent: EP1650215 A1, 2006 ; Location in patent: Page/Page column 7 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2923900090 |
|---|---|
| Summary | 2923900090 other quaternary ammonium salts and hydroxides。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Polyoxometalates--potent and selective ecto-nucleotidase inhibitors.
Biochem. Pharmacol. 93(2) , 171-81, (2015) Polyoxometalates (POMs) are inorganic cluster metal complexes that possess versatile biological activities, including antibacterial, anticancer, antidiabetic, and antiviral effects. Their mechanisms o... |
|
|
Bioactive lysophospholipids generated by hepatic lipase degradation of lipoproteins lead to complement activation via the classical pathway.
Invest. Ophthalmol. Vis. Sci. 55(10) , 6187-93, (2014) We determined bioactivity of lysophospholipids generated by degradation of the low-density (LDL), very low-density (VLDL), and high-density (HDL) lipoproteins with hepatic lipase (HL), cholesterol est... |
|
|
Characterization of lysophospholipid metabolizing enzymes in human brain.
J. Neurochem. 63 , 1839-1848, (1994) Lysophospholipids are generated during the turnover and breakdown of membrane phospholipids. We have identified and partially characterized three enzymes involved in the metabolism of lysophospholipid... |
| 1--(9Z-octadecenoyl)-sn-glycero-3-phosphocholine |
| L-α-LYSOPHOSPHATIDYLCHOLINE, OLEOYL |
| 1-oleoyl-2-hydroxy-sn-glycero-3-phosphocholine |