2-Amino-3-methylbutan-1-ol
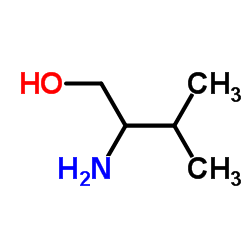
2-Amino-3-methylbutan-1-ol structure
|
Common Name | 2-Amino-3-methylbutan-1-ol | ||
|---|---|---|---|---|
| CAS Number | 16369-05-4 | Molecular Weight | 103.163 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 186.8±13.0 °C at 760 mmHg | |
| Molecular Formula | C5H13NO | Melting Point | 31-32℃ | |
| MSDS | Chinese USA | Flash Point | 91.1±0.0 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
| Name | DL-Valinol |
|---|---|
| Synonym | More Synonyms |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 186.8±13.0 °C at 760 mmHg |
| Melting Point | 31-32℃ |
| Molecular Formula | C5H13NO |
| Molecular Weight | 103.163 |
| Flash Point | 91.1±0.0 °C |
| Exact Mass | 103.099716 |
| PSA | 46.25000 |
| LogP | -0.08 |
| Vapour Pressure | 0.2±0.8 mmHg at 25°C |
| Index of Refraction | 1.447 |
| Storage condition | Refrigerator (+4°C) |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | 36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | UN 2735 |
| WGK Germany | 3 |
| HS Code | 2922199090 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |
| HS Code | 2922199090 |
|---|---|
| Summary | 2922199090. other amino-alcohols, other than those containing more than one kind of oxygen function, their ethers and esters; salts thereof. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Chiral bis(amino alcohol)oxalamide gelators-gelation properties and supramolecular organization: racemate versus pure enantiomer gelation.
Chemistry 9(22) , 5567-80, (2003) Four new chiral bis(amino alcohol)oxalamides (1-4: amino alcohol=leucinol, valinol, phenylglycinol, and phenylalaninol, respectively) have been prepared as low-molecular-weight organic gelators. Their... |
|
|
Synthesis of highly enantioenriched chiral alpha-aminoorganotins via diastereoselective ring opening of chiral N-(arenesulfonyl) 2-tributylstannyloxazolidines.
J. Org. Chem. 74(16) , 5822-38, (2009) trans-N-(Arenesulfonyl)-2-tributylstannyloxazolidines derived from (R)-phenylglycinol were diastereoselectively ring-opened by soft organometallic reagents in the presence of BF(3).OEt(2). Both higher... |
|
|
General synthesis route to benanomicin-pradimicin antibiotics.
Chemistry 13(35) , 9791-823, (2007) A general approach to the regio- and stereoselective total synthesis of the benanomicin-pradimicin antibiotics (BpAs) is described. Construction of the aglycon has been achieved by 1) the diastereosel... |
| 2-amino-3-methylbutan-1-ol |
| 1-Butanol, 2-amino-3-methyl- |
| DL-2-Amino-3-methyl-1-butanol |
| QVYZY1&1 |
| EINECS 240-425-0 |
| valinol |
| 1-Butanol, 2-amino-3-methyl-, |
| MFCD00004730 |
| 2-Amino-3-methyl-1-butanol |
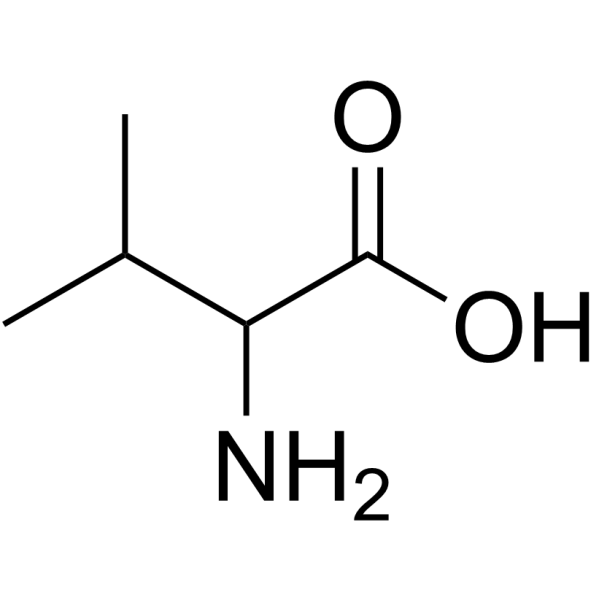 CAS#:516-06-3
CAS#:516-06-3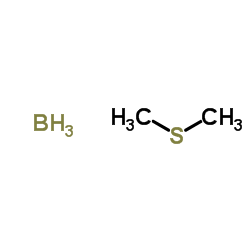 CAS#:13292-87-0
CAS#:13292-87-0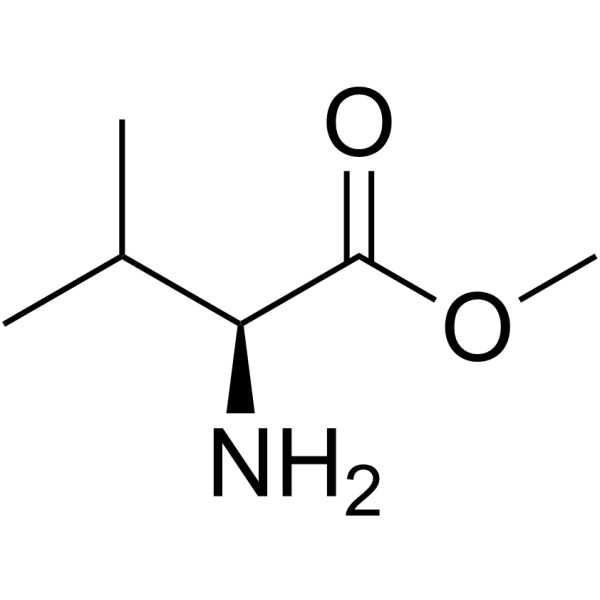 CAS#:4070-48-8
CAS#:4070-48-8 CAS#:17016-83-0
CAS#:17016-83-0 CAS#:16940-66-2
CAS#:16940-66-2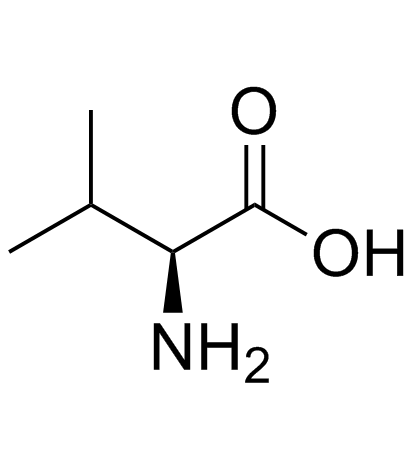 CAS#:72-18-4
CAS#:72-18-4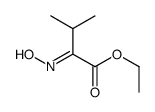 CAS#:77426-65-4
CAS#:77426-65-4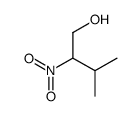 CAS#:77392-54-2
CAS#:77392-54-2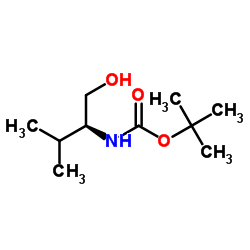 CAS#:79069-14-0
CAS#:79069-14-0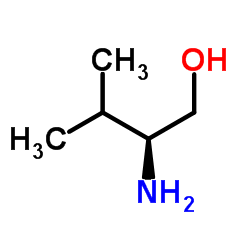 CAS#:4276-09-9
CAS#:4276-09-9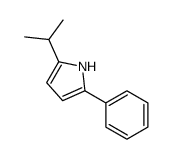 CAS#:13713-07-0
CAS#:13713-07-0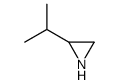 CAS#:13639-42-4
CAS#:13639-42-4 CAS#:169556-48-3
CAS#:169556-48-3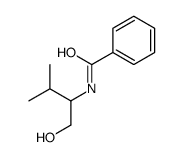 CAS#:92265-07-1
CAS#:92265-07-1![[2-(4-propan-2-yl-4,5-dihydro-1,3-oxazol-2-yl)phenyl]methanol structure](https://image.chemsrc.com/caspic/215/63285-64-3.png) CAS#:63285-64-3
CAS#:63285-64-3 CAS#:4146-21-8
CAS#:4146-21-8
