1-Aminobenzotriazole
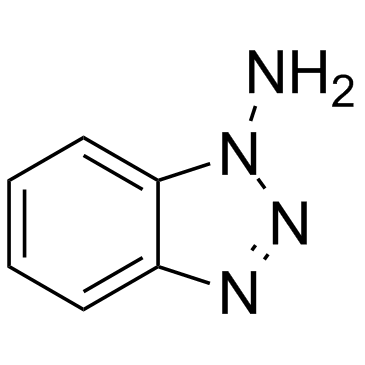
1-Aminobenzotriazole structure
|
Common Name | 1-Aminobenzotriazole | ||
|---|---|---|---|---|
| CAS Number | 1614-12-6 | Molecular Weight | 134.139 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | 319.3±25.0 °C at 760 mmHg | |
| Molecular Formula | C6H6N4 | Melting Point | 81-84 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 146.9±23.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 1-Aminobenzotriazole1-Aminobenzotriazole is a nonspecific and irreversible inhibitor of cytochrome P450 (P450). |
| Name | 1-Aminobenzotriazole |
|---|---|
| Synonym | More Synonyms |
| Description | 1-Aminobenzotriazole is a nonspecific and irreversible inhibitor of cytochrome P450 (P450). |
|---|---|
| Related Catalog | |
| Target |
P450[1] |
| In Vitro | 1-Aminobenzotriazole (ABT) alone significantly increases the expression levels of CYP2B6 in two different hepatocytes (7.3- and 10.8-fold, respectively). Upon co-treatment with 1-Aminobenzotriazole, the induction of CYP2B6 expression by CITCO or rifampin is potentiated: 12.6- and 4.0-fold for CITCO as well as 3.9- and 2.5-fold for rifampin. 1-Aminobenzotriazole has a greater potentiation effect on CITCO than on rifampin. 1-Aminobenzotriazole alone increases the expression levels of CYP3A4 in tow different hepatocytes (by 2.0- and 3.8-fold). Upon co-treatment with 1-Aminobenzotriazole, the effects of CITCO on CYP3A4 expression levels are potentiated by 3.8- and 6.0- fold as compare to cells treated with CITCO alone[1]. 1-Aminobenzotriazole (ABT) (1 mM) shows pronounced (~95%) inhibition of the formation of N-acetylprocainamide compare with the control without 1-Aminobenzotriazole[2]. |
| In Vivo | Oral 1-Aminobenzotriazole (ABT) (100 mg/kg, 2 h predose) decreases the clearance of intravenous procainamide (45%) in rats, accompanied by a decreased N-acetylprocainamide-to-procainamide ratio in urine (0.74 versus 0.21) and plasma (area under the curve ratio 0.59 versus 0.11). The urinary recovery of procainamide increases from 18 to 30%, whereas the recovery of N-acetylprocainamide in urine decreases from 13.3 to 6.5% with 1-Aminobenzotriazole[2]. Pretreatment of rats with 100 mg/kg oral 1-Aminobenzotriazole (ABT) administered 2 hours before a semisolid caloric test meal markedly delays gastric emptying. 1-Aminobenzotriazole also increases stomach weights by 2-fold[3]. |
| Cell Assay | Freshly isolated human hepatocytes are used in this study. Briefly, hepatocytes are placed in serum-free Williams’ E media containing 0.1 μM dexamethasone, 10 μg/mL gentamicin, 15 mM HEPES, 2 mM L-glutamine, and 1% ITS. Cells are incubated for 10 hr at 37°C in an atmosphere containing 5% CO2. After recovery, the hepatocytes are treated with media containing CITCO (100 nM), rifampin (10 μM) or vehicle (ethanol), with or without 1-Aminobenzotriazole (ABT) (1 mM) for 72 hr[1]. |
| Animal Admin | Male Sprague-Dawley rats (0.26 to 0.30 kg, n=3 per treatment) receive an oral dose of 1-Aminobenzotriazole (ABT) (100 mg/kg, 2 mL/kg) 2 h before a single intravenous bolus of procainamide (10 mg/kg, 2 mL/kg). The control group receives only the intravenous bolus of procainamide without 1-Aminobenzotriazole pretreatment. The vehicle for both 1-Aminobenzotriazole and procainamide is 10% dimethylacetamide/90% water (v/v). Rats are fed 4 h after dosing, and serial blood samples are collected at 0.03, 0.17, 0.25, 0.5, 1, 2, 4, and 6 h postdose. Blood samples are centrifuged using tubes containing K3-EDTA as the anticoagulant to obtain plasma. Urine samples are also collected over 24 h postdose. Plasma and urine samples are frozen at -20°C until analysis[2]. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 319.3±25.0 °C at 760 mmHg |
| Melting Point | 81-84 °C(lit.) |
| Molecular Formula | C6H6N4 |
| Molecular Weight | 134.139 |
| Flash Point | 146.9±23.2 °C |
| Exact Mass | 134.059250 |
| PSA | 56.73000 |
| LogP | 0.75 |
| Vapour Pressure | 0.0±0.7 mmHg at 25°C |
| Index of Refraction | 1.774 |
| Water Solubility | slightly soluble |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| Precursor 1 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Chemical genetics reveals a complex functional ground state of neural stem cells.
Nat. Chem. Biol. 3(5) , 268-273, (2007) The identification of self-renewing and multipotent neural stem cells (NSCs) in the mammalian brain holds promise for the treatment of neurological diseases and has yielded new insight into brain canc... |
|
|
Morphological behaviour and metabolic capacity of cryopreserved human primary hepatocytes cultivated in a perfused multiwell device.
Xenobiotica 45(1) , 29-44, (2014) 1. The quantitative prediction of the pharmacokinetic parameters of a drug from data obtained using human in vitro systems remains a significant challenge i.e. prediction of metabolic clearance in hum... |
|
|
Quantitative assessment of intestinal first-pass metabolism of oral drugs using portal-vein cannulated rats.
Pharm. Res. 32(2) , 604-16, (2015) To evaluate the impact of intestinal first-pass metabolism (Fg) by cytochrome P4503A (CYP3A) and uridine 5'-diphosphate-glucuronosyltransferases (UGT) on in vivo oral absorption of their substrate dru... |
| MFCD00132902 |
| 1-Benzotriazolamine,ABT |
| benzotriazol-1-amine |
| 1H-1,2,3-Benzotriazol-1-amine |
| 1-Benzotriazolamine |
| 1H-Benzo[d][1,2,3]triazol-1-amine |
| 1-Aminobenzotriazole |
| 1H-Benzotriazol-1-amine |
 CAS#:95-14-7
CAS#:95-14-7 CAS#:105026-59-3
CAS#:105026-59-3 CAS#:259-79-0
CAS#:259-79-0 CAS#:122-79-2
CAS#:122-79-2 CAS#:796-30-5
CAS#:796-30-5![1,4-diphenyl-2,3-benzo-7,7,8,8-tetramethyl-7,8-digermabicyclo[2,2,2]octadiene structure](https://image.chemsrc.com/caspic/383/84784-54-3.png) CAS#:84784-54-3
CAS#:84784-54-3![3a,4,9,9a-tetrahydro-2-methyl-4,9-diphenyl-4,9-epoxybenzo[f]isoindole-1,3-dione structure](https://image.chemsrc.com/caspic/211/108346-08-3.png) CAS#:108346-08-3
CAS#:108346-08-3 CAS#:1159-86-0
CAS#:1159-86-0 CAS#:751-38-2
CAS#:751-38-2 CAS#:615-42-9
CAS#:615-42-9 CAS#:5471-63-6
CAS#:5471-63-6
