2-Amino-6-bromobenzothiazole
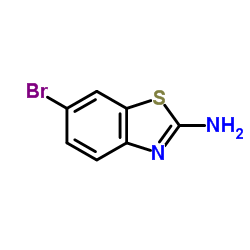
2-Amino-6-bromobenzothiazole structure
|
Common Name | 2-Amino-6-bromobenzothiazole | ||
|---|---|---|---|---|
| CAS Number | 15864-32-1 | Molecular Weight | 229.10 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 366.8±34.0 °C at 760 mmHg | |
| Molecular Formula | C7H5BrN2S | Melting Point | 213-217 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 175.7±25.7 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Amino-6-bromobenzothiazole2-Amino-6-bromobenzothiazole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 2-Amino-6-bromobenzothiazole |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Amino-6-bromobenzothiazole is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 366.8±34.0 °C at 760 mmHg |
| Melting Point | 213-217 °C(lit.) |
| Molecular Formula | C7H5BrN2S |
| Molecular Weight | 229.10 |
| Flash Point | 175.7±25.7 °C |
| Exact Mass | 227.935669 |
| PSA | 67.15000 |
| LogP | 2.66 |
| Vapour Pressure | 0.0±0.8 mmHg at 25°C |
| Index of Refraction | 1.783 |
| Storage condition | Room temperature. |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H317-H319 |
| Precautionary Statements | P280-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | Xn:Harmful; |
| Risk Phrases | R22;R36;R43 |
| Safety Phrases | S26-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2934999090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Prokaryotic NavMs channel as a structural and functional model for eukaryotic sodium channel antagonism.
Proc. Natl. Acad. Sci. U. S. A. 111(23) , 8428-33, (2014) Voltage-gated sodium channels are important targets for the development of pharmaceutical drugs, because mutations in different human sodium channel isoforms have causal relationships with a range of ... |
|
|
Efficient synthesis of 2-amino-6-arylbenzothiazoles via Pd (0) Suzuki cross coupling reactions: potent urease enzyme inhibition and nitric oxide scavenging activities of the products. Gull Y, et al.
Molecules 18(8) , 8845-8857, (2013)
|
|
|
Synthesis, cytostatic, and antitumor properties of new Rh (I) thiazole complexes. Craciunescu, D. G., et al.
Biol. Trace Elem. Res. 8(4) , 251-261, (1985)
|
| 2-Amino-6-bromobenzothiazole |
| MFCD00152229 |
| 2-Amino-6-Bromo benthiazole |
| 6-Bromobenzo[d]thiazol-2-amine |
| 2-Benzothiazolamine, 6-bromo- |
| 6-Bromo-1,3-benzothiazol-2-amine |
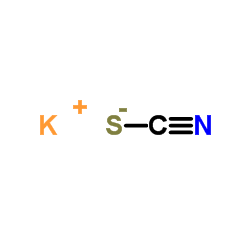 CAS#:333-20-0
CAS#:333-20-0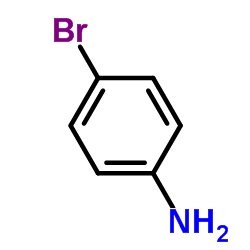 CAS#:106-40-1
CAS#:106-40-1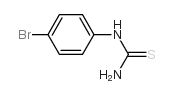 CAS#:2646-30-2
CAS#:2646-30-2![Benzo[d]thiazol-2-amine Structure](https://image.chemsrc.com/caspic/040/136-95-8.png) CAS#:136-95-8
CAS#:136-95-8 CAS#:21327-14-0
CAS#:21327-14-0 CAS#:3674-54-2
CAS#:3674-54-2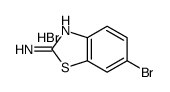 CAS#:63450-94-2
CAS#:63450-94-2 CAS#:624-19-1
CAS#:624-19-1 CAS#:540-72-7
CAS#:540-72-7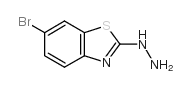 CAS#:37390-63-9
CAS#:37390-63-9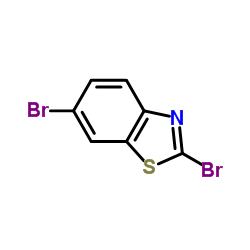 CAS#:408328-13-2
CAS#:408328-13-2![6-Bromobenzo[d]thiazole structure](https://image.chemsrc.com/caspic/458/53218-26-1.png) CAS#:53218-26-1
CAS#:53218-26-1![2-[(2-amino-5-bromophenyl)disulfanyl]-4-bromoaniline structure](https://image.chemsrc.com/caspic/396/182499-80-5.png) CAS#:182499-80-5
CAS#:182499-80-5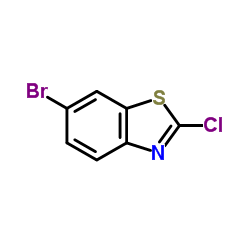 CAS#:80945-86-4
CAS#:80945-86-4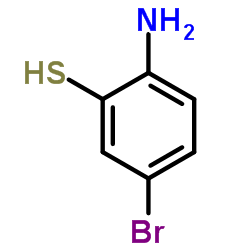 CAS#:23451-95-8
CAS#:23451-95-8 CAS#:131395-09-0
CAS#:131395-09-0 CAS#:3566-95-8
CAS#:3566-95-8 CAS#:3427-31-4
CAS#:3427-31-4![4,6-Dibromobenzo[d]thiazol-2-amine structure](https://image.chemsrc.com/caspic/064/16582-60-8.png) CAS#:16582-60-8
CAS#:16582-60-8
