L-DON

L-DON structure
|
Common Name | L-DON | ||
|---|---|---|---|---|
| CAS Number | 157-03-9 | Molecular Weight | 171.15400 | |
| Density | 1.3994 (rough estimate) | Boiling Point | 301.12°C (rough estimate) | |
| Molecular Formula | C6H9N3O3 | Melting Point | -145ºC (dec.) | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of L-DONL-6-Diazo-5-oxonorleucine (L-6-Diazo-5-oxonorleucine) is a glutaminases antagonist with a Ki of 6 μM. L-6-Diazo-5-oxonorleucine exhibits analgesic, antibacterial, antiviral and anticancer properties. L-6-Diazo-5-oxonorleucine displays genetic toxicity in vitro. L-6-Diazo-5-oxonorleucine decreases the self-renewal potential and metastatic ability of tumor cell[1][2][3][4]. |
| Name | 6-Diazo-5-oxo-L-norleucine |
|---|---|
| Synonym | More Synonyms |
| Description | L-6-Diazo-5-oxonorleucine (L-6-Diazo-5-oxonorleucine) is a glutaminases antagonist with a Ki of 6 μM. L-6-Diazo-5-oxonorleucine exhibits analgesic, antibacterial, antiviral and anticancer properties. L-6-Diazo-5-oxonorleucine displays genetic toxicity in vitro. L-6-Diazo-5-oxonorleucine decreases the self-renewal potential and metastatic ability of tumor cell[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Ki: 6 μM (glutaminases)[4] |
| References |
| Density | 1.3994 (rough estimate) |
|---|---|
| Boiling Point | 301.12°C (rough estimate) |
| Melting Point | -145ºC (dec.) |
| Molecular Formula | C6H9N3O3 |
| Molecular Weight | 171.15400 |
| Exact Mass | 171.06400 |
| PSA | 117.78000 |
| Appearance of Characters | crystalline | light yellow |
| Index of Refraction | 1.5800 (estimate) |
| Storage condition | −20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H311-H331 |
| Precautionary Statements | P261-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T |
| Risk Phrases | 23/24/25 |
| Safety Phrases | S45;S36/S37/S39 |
| RIDADR | UN 2811 6.1/PG 3 |
| WGK Germany | 3 |
| RTECS | RC6340000 |
| HS Code | 2927000090 |
| HS Code | 2927000090 |
|---|---|
| Summary | 2927000090 other diazo-, azo- or azoxy-compounds。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Glutamate secretion and metabotropic glutamate receptor 1 expression during Kaposi's sarcoma-associated herpesvirus infection promotes cell proliferation.
PLoS Pathog. 10(10) , e1004389, (2014) Kaposi's sarcoma associated herpesvirus (KSHV) is etiologically associated with endothelial Kaposi's sarcoma (KS) and B-cell proliferative primary effusion lymphoma (PEL), common malignancies seen in ... |
|
|
GMP synthase is essential for viability and infectivity of Trypanosoma brucei despite a redundant purine salvage pathway.
Mol. Microbiol. 97 , 1006-20, (2015) The causative agent of human African trypanosomiasis, Trypanosoma brucei, lacks de novo purine biosynthesis and depends on purine salvage from the host. The purine salvage pathway is redundant and con... |
|
|
Gemcitabine diphosphate choline is a major metabolite linked to the Kennedy pathway in pancreatic cancer models in vivo.
Br. J. Cancer 111(2) , 318-25, (2014) The modest benefits of gemcitabine (dFdC) therapy in patients with pancreatic ductal adenocarcinoma (PDAC) are well documented, with drug delivery and metabolic lability cited as important contributin... |
| (S)-2-Amino-6-diazo-5-oxocaproic acid,DON |
| 6-DIAZO-5-OXO-L-NORLEUCINE |
| MFCD00037218 |
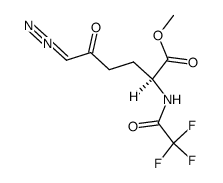 CAS#:7589-24-4
CAS#:7589-24-4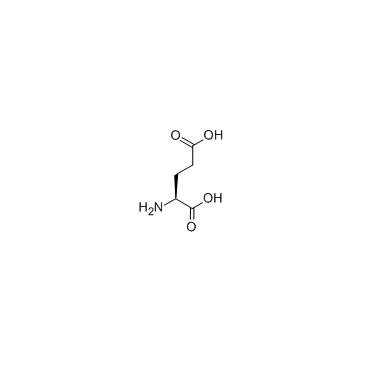 CAS#:56-86-0
CAS#:56-86-0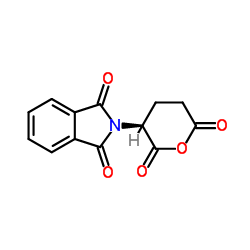 CAS#:25830-77-7
CAS#:25830-77-7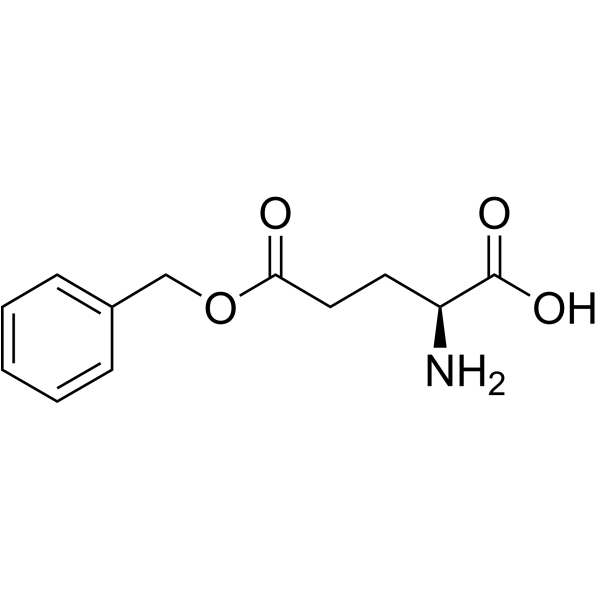 CAS#:1676-73-9
CAS#:1676-73-9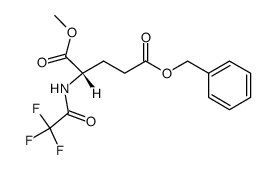 CAS#:84369-01-7
CAS#:84369-01-7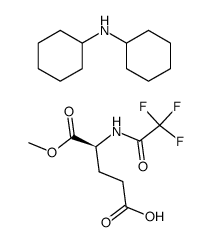 CAS#:84344-29-6
CAS#:84344-29-6 CAS#:1535-57-5
CAS#:1535-57-5 CAS#:71989-18-9
CAS#:71989-18-9