4-[(3β)-Cholest-5-en-3-yloxy]-4-oxobutanoic acid
![4-[(3β)-Cholest-5-en-3-yloxy]-4-oxobutanoic acid Structure](https://image.chemsrc.com/caspic/456/1510-21-0.png)
4-[(3β)-Cholest-5-en-3-yloxy]-4-oxobutanoic acid structure
|
Common Name | 4-[(3β)-Cholest-5-en-3-yloxy]-4-oxobutanoic acid | ||
|---|---|---|---|---|
| CAS Number | 1510-21-0 | Molecular Weight | 486.73 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 586.0±43.0 °C at 760 mmHg | |
| Molecular Formula | C31H50O4 | Melting Point | 178 °C | |
| MSDS | Chinese USA | Flash Point | 179.6±21.7 °C | |
Use of 4-[(3β)-Cholest-5-en-3-yloxy]-4-oxobutanoic acidCholesteryl hemisuccinate is a with hepatoprotective an anticancer activity. Cholesteryl hemisuccinate inhibits Acetaminophen (AAP, HY-66005) hepatotoxicity, and prevents AAP-induced hepatic apoptosis and necrosis. Cholesteryl hemisuccinate inhibits DNA polymerase and DNA topoisomerase to inhibit DNA replication and repair and cell division. Thus, Cholesteryl hemisuccinate inhibits tumor growth[1][2]. |
| Name | Cholesteryl hemisuccinate |
|---|---|
| Synonym | More Synonyms |
| Description | Cholesteryl hemisuccinate is a with hepatoprotective an anticancer activity. Cholesteryl hemisuccinate inhibits Acetaminophen (AAP, HY-66005) hepatotoxicity, and prevents AAP-induced hepatic apoptosis and necrosis. Cholesteryl hemisuccinate inhibits DNA polymerase and DNA topoisomerase to inhibit DNA replication and repair and cell division. Thus, Cholesteryl hemisuccinate inhibits tumor growth[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Cholesteryl hemisuccinate(100 mg/kg;ip;AAP 前单次给药)消除 AAP(350-500 mg/kg;ip;单次给药)在 ICR 小鼠 (CD-1) 中诱导的细胞凋亡和坏死的组织学和生化诊断[1]。 |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 586.0±43.0 °C at 760 mmHg |
| Melting Point | 178 °C |
| Molecular Formula | C31H50O4 |
| Molecular Weight | 486.73 |
| Flash Point | 179.6±21.7 °C |
| Exact Mass | 486.370911 |
| PSA | 63.60000 |
| LogP | 10.32 |
| Vapour Pressure | 0.0±3.5 mmHg at 25°C |
| Index of Refraction | 1.529 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
|
Co-expression of human agouti-related protein enhances expression and stability of human melanocortin-4 receptor.
Biochem. Biophys. Res. Commun. 456(1) , 116-21, (2014) G protein-coupled receptors (GPCRs) represent the largest family of transmembrane signaling proteins, and they are considered major targets of approximately half of all therapeutic agents. Human melan... |
|
|
Solution structure of the transmembrane 2 domain of the human melanocortin-4 receptor in sodium dodecyl sulfate (SDS) micelles and the functional implication of the D90N mutant.
Biochim. Biophys. Acta 1848 , 1294-302, (2015) The melanocortin receptors (MCRs) are members of the G protein-coupled receptor (GPCR) 1 superfamily with seven transmembrane (TM) domains. Among them, the melanocortin-4 receptor (MC4R) subtype has b... |
|
|
Structural basis for the facilitative diffusion mechanism by SemiSWEET transporter.
Nat. Commun. 6 , 6112, (2015) SWEET family proteins mediate sugar transport across biological membranes and play crucial roles in plants and animals. The SWEETs and their bacterial homologues, the SemiSWEETs, are related to the PQ... |
| 4-[(3β)-Cholest-5-en-3-yloxy]-4-oxobutanoic acid |
| 3-cholesteryloxycarbonylpropanoic acid |
| Cholesteryl hydrogen succinate |
| EINECS 216-148-6 |
| 3-(3-cholesteryloxycarbonyl)propanoic acid |
| MONO-CHOLESTERYL-SUCCINATE |
| CHOLESTEROL HYDROGEN SUCCINATE |
| 3β-Hydroxy-5-cholestene 3-hemisuccinate |
| MFCD00037705 |
| cholesteryl hemisuccinate free acid |
| cholesterylsuccinate |
| 5-Cholesten-3β-ol 3-hemisuccinate |
| Succinic Acid Monocholesterol Ester |
| 5-CHOLESTEN-3BETA-OL 3-HEMISUCCINATE |
| Butanedioic acid, mono[(3β)-cholest-5-en-3-yl] ester |
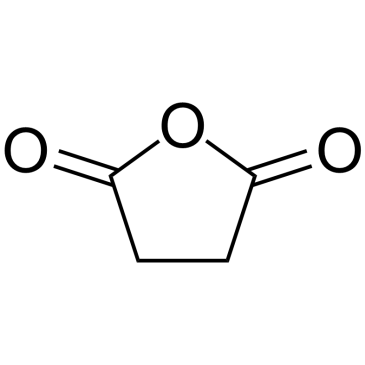 CAS#:108-30-5
CAS#:108-30-5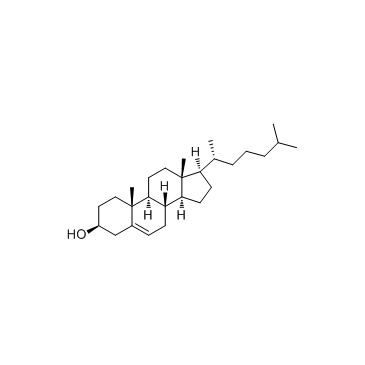 CAS#:57-88-5
CAS#:57-88-5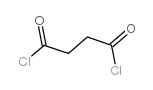 CAS#:543-20-4
CAS#:543-20-4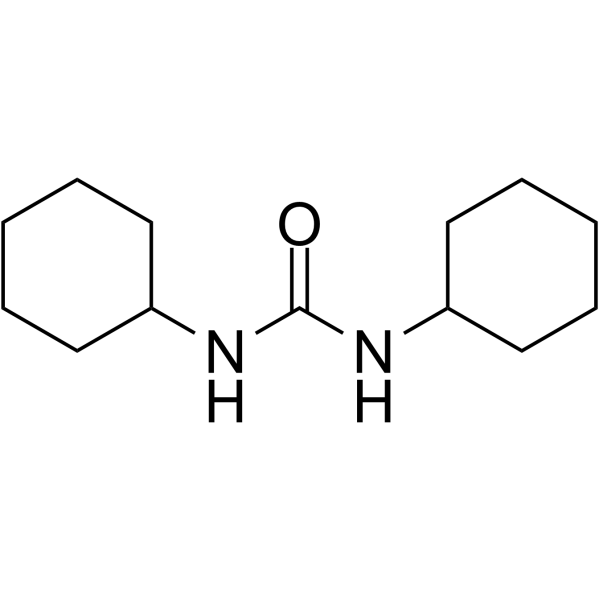 CAS#:2387-23-7
CAS#:2387-23-7
