2,4,6-Tribromoaniline
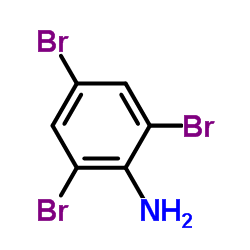
2,4,6-Tribromoaniline structure
|
Common Name | 2,4,6-Tribromoaniline | ||
|---|---|---|---|---|
| CAS Number | 147-82-0 | Molecular Weight | 329.815 | |
| Density | 2.4±0.1 g/cm3 | Boiling Point | 300.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C6H4Br3N | Melting Point | 120-122 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 132.9±25.9 °C | |
| Symbol |



GHS05, GHS06, GHS08 |
Signal Word | Danger | |
| Name | 2,4,6-Tribromoaniline |
|---|---|
| Synonym | More Synonyms |
| Density | 2.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 300.0±0.0 °C at 760 mmHg |
| Melting Point | 120-122 °C(lit.) |
| Molecular Formula | C6H4Br3N |
| Molecular Weight | 329.815 |
| Flash Point | 132.9±25.9 °C |
| Exact Mass | 326.789368 |
| PSA | 26.02000 |
| LogP | 4.43 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.689 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS05, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301 + H311 + H331-H315-H318-H373 |
| Precautionary Statements | P261-P280-P301 + P310-P305 + P351 + P338-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R23/24/25;R33 |
| Safety Phrases | S28-S36/37/39-S45-S36/37-S28A-S26 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 3 |
| RTECS | BZ0175000 |
| Packaging Group | I; II; III |
| Hazard Class | 6.1 |
| HS Code | 2921420090 |
| Precursor 9 | |
|---|---|
| DownStream 9 | |
| HS Code | 2921420090 |
|---|---|
| Summary | HS:2921420090 aniline derivatives and their salts VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:30.0% |
|
Methods for the determination of phenolic brominated flame retardants, and by-products, formulation intermediates and decomposition products of brominated flame retardants in water
J. Chromatogr. A. 1216(3) , 334-45, (2009) Brominated flame retardants (BFRs) are the chemicals of high importance within the REAch framework. In addition to polybrominated diphenyl ethers (PBDEs), hexabromocyclododecane (HBCD) and tetrabromob... |
|
|
Voltammetric study of the oxidation kinetics of 2, 4, 6,-tribromoaniline in acid medium. Brillas E, et al.
J. Electroanal. Chem. Interfac. Electrochem. 160(1) , 185-198, (1984)
|
|
|
Formation of bromodioxin analogs from closed tube pyrolysis of 2, 4, 6-tribromoaniline (I). Alsabbagh AM, et al.
Chemosphere 17(12) , 2391-96, (1988)
|
| 2,4,6-tribromobenzeneamine |
| 2,4,6-Tribromobenzenamine |
| Benzenamine,2,4,6-tribromo |
| ZR BE DE FE |
| Aniline,2,4,6-tribromo |
| 2,4,6-Tribromoaniline |
| 2,3-DIHYDRO-BENZO[1,4]DIOXINE-6-SULFONIC ACID (3-CHLORO-QUINOXALIN-2-YL)-AMIDE |
| sym-Tribromoaniline |
| EINECS 205-700-1 |
| MFCD00007634 |
| Aniline tribromide |
| s-Tribromoaniline |
| 2,4,6-tribromo-aniline |
| 2,4,6-Tribromophenylamine |
| Benzenamine, 2,4,6-tribromo- |
| 2,4,6-tribromaniline |
| (2,4,6-Tribromophenyl)amine |
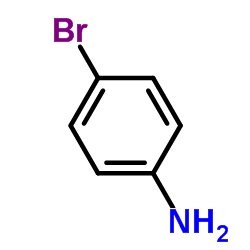 CAS#:106-40-1
CAS#:106-40-1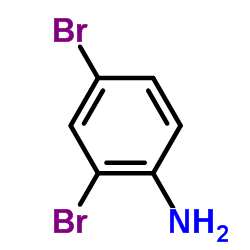 CAS#:615-57-6
CAS#:615-57-6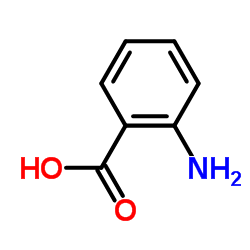 CAS#:118-92-3
CAS#:118-92-3 CAS#:1972-28-7
CAS#:1972-28-7 CAS#:615-36-1
CAS#:615-36-1 CAS#:121-57-3
CAS#:121-57-3 CAS#:412027-33-9
CAS#:412027-33-9 CAS#:19617-84-6
CAS#:19617-84-6 CAS#:26733-18-6
CAS#:26733-18-6 CAS#:103068-20-8
CAS#:103068-20-8 CAS#:488-48-2
CAS#:488-48-2 CAS#:2973-00-4
CAS#:2973-00-4 CAS#:634-89-9
CAS#:634-89-9 CAS#:88-89-1
CAS#:88-89-1 CAS#:291539-77-0
CAS#:291539-77-0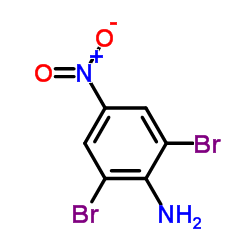 CAS#:827-94-1
CAS#:827-94-1 CAS#:874-17-9
CAS#:874-17-9 CAS#:6864-20-6
CAS#:6864-20-6
