Ac-Tyr-Val-Ala-Asp-aldehyde (pseudo acid)
Modify Date: 2025-08-22 20:13:00
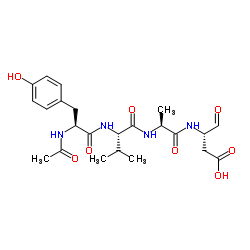
Ac-Tyr-Val-Ala-Asp-aldehyde (pseudo acid) structure
|
Common Name | Ac-Tyr-Val-Ala-Asp-aldehyde (pseudo acid) | ||
|---|---|---|---|---|
| CAS Number | 143313-51-3 | Molecular Weight | 492.522 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 937.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C23H32N4O8 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 520.9±34.3 °C | |
Use of Ac-Tyr-Val-Ala-Asp-aldehyde (pseudo acid)Ac-YVAD-CHO (L-709049) is a potent, reversible, specific tetrapeptide interleukin-lβ converting enzyme (ICE) inhibitor with mouse and human Ki values of 3.0 and 0.76 nM. Ac-YVAD-CHO can suppress the production of mature IL-lβ[1]. |
| Name | ac-yvad-cho |
|---|---|
| Synonym | More Synonyms |
| Description | Ac-YVAD-CHO (L-709049) is a potent, reversible, specific tetrapeptide interleukin-lβ converting enzyme (ICE) inhibitor with mouse and human Ki values of 3.0 and 0.76 nM. Ac-YVAD-CHO can suppress the production of mature IL-lβ[1]. |
|---|---|
| Related Catalog | |
| Target |
IL-1β:3 nM (Ki) IL-1β:0.76 nM (Ki) |
| In Vitro | Ac-YVAD-CHO inhibits IL-1β in a dose-dependent manner, with mouse and human IC50 values of 2.5 and 0.7 μM. Ac-YVAD-CHO (0.01~100 μM) reduces the elevations of IL-lβ in the plasma and peritoneal fluid treated with LPS in a dose-related manner[1]. |
| In Vivo | Ac-YVAD-CHO (10 mg/kg; i.p.; 1 hour) is absorbed from the peritoneal cavity, as well as cleared from the blood rapidly[1]. Ac-YVAD-CHO (50 mg/kg; i.p.; 1 hour) drops precipitously to approximately 1 and 0.2 μM at 30 and 60 minutes after injection[1]. Ac-YVAD-CHO (30 mg/kg; i.p.; 6 hours) suppresses IL-1β levels[1]. Animal Model: CD1 female mice Dosage: 10 mg/kg (Pharmacokinetic Analysis) Administration: I.p.; 1 hour Result: Absorbed from the peritoneal cavity, as well as cleared from the blood rapidly. Animal Model: CD1 female mice Dosage: 50 mg/kg (Pharmacokinetic Analysis) Administration: I.p.; 1 hour Result: Dropped precipitously to approximately 1 and 0.2 μM at 30 and 60 minutes after injection. Animal Model: P. acnes-sensitized mice Dosage: 30 mg/kg Administration: I.p.; 6 hours Result: Suppressed IL-1β levels. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 937.7±65.0 °C at 760 mmHg |
| Molecular Formula | C23H32N4O8 |
| Molecular Weight | 492.522 |
| Flash Point | 520.9±34.3 °C |
| Exact Mass | 492.222015 |
| PSA | 191.00000 |
| LogP | 0.79 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | −20°C |
| WGK Germany | 3 |
|---|
| pase inhibitor i |
| acetyl-yvad-aldehyde |
| ice inhibitor |
| ac-yvad-aldehyde |
| N-Acetyl-L-tyrosyl-L-valyl-N-[(2S)-1-carboxy-3-oxopropan-2-yl]-L-alaninamide |
| L-Alaninamide, N-acetyl-L-tyrosyl-L-valyl-N-((1S)-2-carboxy-1-formylethyl)- |
| tyr-val-ala-asp-cho |
| N-Acetyl-L-tyrosyl-L-valyl-N-[(2S)-1-carboxy-3-oxo-2-propanyl]-L-alaninamide |
| yvad-cho |
| L-Alaninamide, N-acetyl-L-tyrosyl-L-valyl-N-[(1S)-2-carboxy-1-formylethyl]- |
| ice inhibitor i |

