5-(N,N-Hexamethylene)-amiloride
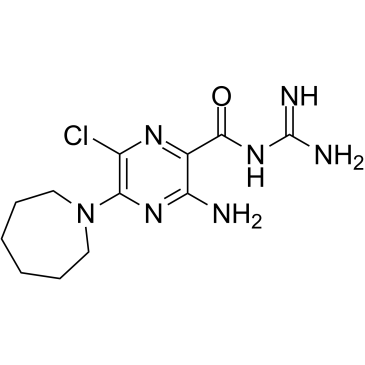
5-(N,N-Hexamethylene)-amiloride structure
|
Common Name | 5-(N,N-Hexamethylene)-amiloride | ||
|---|---|---|---|---|
| CAS Number | 1428-95-1 | Molecular Weight | 311.77100 | |
| Density | 1.63g/cm3 | Boiling Point | 638.2ºC at 760mmHg | |
| Molecular Formula | C12H18ClN7O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 339.8ºC | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of 5-(N,N-Hexamethylene)-amiloride5-(N,N-Hexamethylene)-amiloride (Hexamethylene amiloride) derives from an amiloride and is a potent Na+/H+ exchanger inhibitor, which decreases the intracellular pH (pHi) and induces apoptosis in leukemic cells. 5-(N,N-Hexamethylene)-amiloride (Hexamethylene amiloride) is also an inhibitor of the HIV-1 Vpu virus ion channel and inhibits mouse hepatitis virus (MHV) replication and human coronavirus 229E (HCoV229E) replication in cultured L929 cells with EC50s of 3.91 μM and 1.34 μM, respectively[1][2]. |
| Name | 5-(N,N-hexamethylene)amiloride |
|---|---|
| Synonym | More Synonyms |
| Description | 5-(N,N-Hexamethylene)-amiloride (Hexamethylene amiloride) derives from an amiloride and is a potent Na+/H+ exchanger inhibitor, which decreases the intracellular pH (pHi) and induces apoptosis in leukemic cells. 5-(N,N-Hexamethylene)-amiloride (Hexamethylene amiloride) is also an inhibitor of the HIV-1 Vpu virus ion channel and inhibits mouse hepatitis virus (MHV) replication and human coronavirus 229E (HCoV229E) replication in cultured L929 cells with EC50s of 3.91 μM and 1.34 μM, respectively[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Na+/H+ exchanger[1] EC50: 3.91 μM (MHV replication), 1.34 μM (HCoV229E replication)[2] |
| References |
| Density | 1.63g/cm3 |
|---|---|
| Boiling Point | 638.2ºC at 760mmHg |
| Molecular Formula | C12H18ClN7O |
| Molecular Weight | 311.77100 |
| Flash Point | 339.8ºC |
| Exact Mass | 311.12600 |
| PSA | 136.51000 |
| LogP | 2.55300 |
| Vapour Pressure | 3.47E-16mmHg at 25°C |
| Index of Refraction | 1.742 |
| Storage condition | 2-8°C |
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H301-H311-H331 |
| Precautionary Statements | P261-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T |
| Risk Phrases | 23/24/25 |
| Safety Phrases | 22-36/37/39-45 |
| RIDADR | UN 2811 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2934999090 |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Gossypetin ameliorates ionizing radiation-induced oxidative stress in mice liver--a molecular approach.
Free Radic. Res. 49 , 1173-86, (2015) Radioprotective action of gossypetin (GTIN) against gamma (γ)-radiation-induced oxidative stress in liver was explored in the present article. Our main aim was to evaluate the protective efficacy of G... |
|
|
Differential effects of viroporin inhibitors against feline infectious peritonitis virus serotypes I and II.
Arch. Virol. 160(5) , 1163-70, (2015) Feline infectious peritonitis virus (FIP virus: FIPV), a feline coronavirus of the family Coronaviridae, causes a fatal disease called FIP in wild and domestic cat species. The genome of coronaviruses... |
|
|
Effects of quaternization on the morphological stability and antibacterial activity of electrospun poly(DMAEMA-co-AMA) nanofibers.
Colloids Surf. B Biointerfaces 133 , 148-55, (2015) Electrospun nanofibers with antibacterial activity are greatly promising for medical treatment and water purification. Herein we report antibacterial nanofibers electrospun from a series of poly(dimet... |
| HMA |
| 3-amino-5-azepan-1-yl-6-chloro-pyrazine-2-carboxylic acid carbamimidoylamide |
| HMA-5 |
| 3-amino-5-(azepan-1-yl)-6-chloro-N-(diaminomethylidene)pyrazine-2-carboxamide |
| Hexamethyleneamiloride |