(−)-Conophylline
Modify Date: 2025-08-24 19:24:28
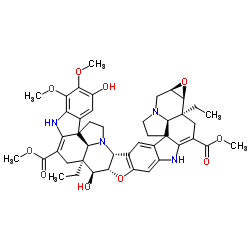
(−)-Conophylline structure
|
Common Name | (−)-Conophylline | ||
|---|---|---|---|---|
| CAS Number | 142741-24-0 | Molecular Weight | 794.889 | |
| Density | 1.5±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C44H50N4O10 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (−)-ConophyllineConophylline is a vinca alkaloid extracted from leaves of a tropical plant Ervatamia microphylla. Conophylline is a differentiation inducer of for pancreatic cells. Conophylline suppresses HSC and induces apoptosis[1][2]. |
| Name | Conophylline |
|---|---|
| Synonym | More Synonyms |
| Description | Conophylline is a vinca alkaloid extracted from leaves of a tropical plant Ervatamia microphylla. Conophylline is a differentiation inducer of for pancreatic cells. Conophylline suppresses HSC and induces apoptosis[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Conophylline (100 ng/ml; 48 hours) reproduces differentiationinducing activity but not apoptosis-inducing activity of activin[1]. Conophylline (100 ng/ml; 72 hours) exhibits the differentiation-inducing activity in AR42J cells and converts these cells to endocrine cells[1]. Conophylline increases the expression of neurogenin-3 by activating p38 mitogen-activated protein kinase to induce differentiation of AR42J cells[1]. Conophylline reduces the expression of α-SMA and collagen-1 in rat HSC and Lx-2 cells[2]. Conophylline inhibits DNA synthesis induced by serum[2]. Conophylline also promots activation of caspase-3 and induces apoptosis in Lx-2 Cells[2]. Apoptosis Analysis[2] Cell Line: Lx-2 Cells Concentration: 12 hours Incubation Time: 0.1 μg/ml Result: Induced apoptosis Western Blot Analysis[2] Cell Line: Lx-2 cells Concentration: 0.1 μg/ml Incubation Time: 15 minutes, 30 minutes, 60 minutes, 120 minutes Result: Increased phospho-JNK. |
| In Vivo | Conophylline (0.9 mg/kg; p.o.; daily; for 12 weeks) attenuates formation of the liver fibrosis induced by TAA in vivo[2]. Animal Model: Sprague-Dawley rats (70-80 g)[2] Dosage: 0.9 mg/kg Administration: Oral administration; daily; for 12 weeks Result: Attenuated formation of the liver fibrosis induced by TAA. |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Molecular Formula | C44H50N4O10 |
| Molecular Weight | 794.889 |
| Exact Mass | 794.352722 |
| PSA | 163.82000 |
| LogP | 4.49 |
| Index of Refraction | 1.728 |
| InChIKey | QZRIMAMDGWAHPQ-ADSDEVAISA-N |
| SMILES | CCC12CC(C(=O)OC)=C3Nc4cc5c(cc4C34CCN(CC3OC31)C42)C1C(O5)C(O)C2(CC)CC(C(=O)OC)=C3Nc4c(cc(O)c(OC)c4OC)C34CCN1C42 |
| 2H,22H-Indolo[2'',3'':7',8']pyrrolo[1'',2'',3'':1',8']quino[2',3':4,5]furo[2,3-b]oxireno[6,7]indolizino[1,8-fg]carbazole-3,9-dicarboxylic acid, 1b,7a-diethyl-1a,1b,4,6a,7,7a,8,10,14c,15,16,17a,19,20,21a,22a-hexadecahydro-7,13-dihydroxy-11,12-dimethoxy-, dimethyl ester, (1aS,1bS,6aS,7S,7aS,14bR,14cR,17aR,18bR,21aR,22aR)- |
| (−)-Conophylline |
| Dimethyl (1aS,1bS,6aS,7S,7aS,14bR,14cR,17aR,18bR,21aR,22aR)-1b,7a-diethyl-7,13-dihydroxy-11,12-dimethoxy-1b,4,6a,7,7a,8,10,14c,15,16,17a,19,20,21a,22,22a-hexadecahydro-1aH,2H-indolo[2'',3'':7',8']pyrrolo[1'',2'',3'':1',8']quinolino[2',3':4,5]furo[2,3-b]oxireno[6,7]indolizino[1,8-fg]carbazole-3,9-dicarboxylate |

