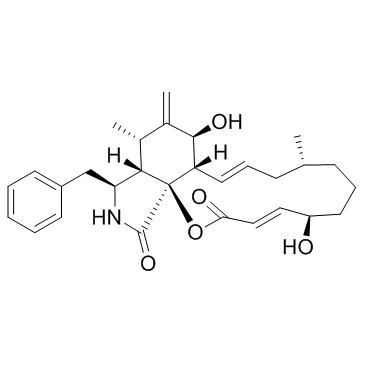
Cytochalasin A

Cytochalasin A structure
|
Common Name | Cytochalasin A | ||
|---|---|---|---|---|
| CAS Number | 14110-64-6 | Molecular Weight | 477.592 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 725.1±60.0 °C at 760 mmHg | |
| Molecular Formula | C29H35NO5 | Melting Point | 147 to 148'ºC | |
| MSDS | USA | Flash Point | 392.3±32.9 °C | |
| Symbol |


GHS06, GHS08 |
Signal Word | Danger | |
Use of Cytochalasin ACytochalasin A is a cell-permeable fungal toxin that is an oxidized derivative of cytochalasin B. Cytochalasin A is an inhibitor of HIV-1 protease (IC50=3 μM) and inhibits actin polymerization and interferes with microtubule assembly by reacting with sulfhydryl groups. Antibiotic and fungicidal activitives[1][2]. |
| Name | cytochalasin a |
|---|---|
| Synonym | More Synonyms |
| Description | Cytochalasin A is a cell-permeable fungal toxin that is an oxidized derivative of cytochalasin B. Cytochalasin A is an inhibitor of HIV-1 protease (IC50=3 μM) and inhibits actin polymerization and interferes with microtubule assembly by reacting with sulfhydryl groups. Antibiotic and fungicidal activitives[1][2]. |
|---|---|
| Related Catalog | |
| Target |
HIV-1 protease[1] |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 725.1±60.0 °C at 760 mmHg |
| Melting Point | 147 to 148'ºC |
| Molecular Formula | C29H35NO5 |
| Molecular Weight | 477.592 |
| Flash Point | 392.3±32.9 °C |
| Exact Mass | 477.251526 |
| PSA | 92.70000 |
| LogP | 2.83 |
| Vapour Pressure | 0.0±2.5 mmHg at 25°C |
| Index of Refraction | 1.590 |
| Storage condition | −20°C |
| Water Solubility | ethanol: 20 mg/mL, clear, colorless | Soluble in DMF, DMSO, ethanol and acetone. Insoluble in water. |
| Symbol |


GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H310-H330-H361 |
| Precautionary Statements | P260-P264-P280-P284-P302 + P350-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic;T: Toxic; |
| Risk Phrases | R26/27/28 |
| Safety Phrases | 28-36/37-45 |
| RIDADR | UN 1544 6.1/PG 2 |
| WGK Germany | 3 |
| Packaging Group | I |
| Hazard Class | 6.1(a) |
| HS Code | 29349990 |
|
~% 
Cytochalasin A CAS#:14110-64-6 |
| Literature: Journal of the American Chemical Society, , vol. 99, # 20 p. 6756 - 6758 |
|
~% 
Cytochalasin A CAS#:14110-64-6 |
| Literature: Helvetica chimica acta, , vol. 53, # 4 p. 696 - 724 |
|
~% 
Cytochalasin A CAS#:14110-64-6 |
| Literature: Journal of the American Chemical Society, , vol. 99, # 20 p. 6756 - 6758 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
|
Apical growth and mitosis are independent processes in Aspergillus nidulans.
Protoplasma 222(3-4) , 211-5, (2003) It is well established that cytoplasmic microtubules are depolymerized during nuclear division and reassembled as mitotic microtubules. Mounting evidence showing that cytoplasmic microtubules were als... |
|
|
Roles of the actin cytoskeleton and an actin-binding protein in wheat resistance against Puccinia striiformis f. sp. tritici.
Protoplasma 249(1) , 99-106, (2012) Elucidating resistance mechanisms of plant cells against pathogens is essential to develop novel strategies of disease control. The actin cytoskeleton was found intimately involved in plant defense. I... |
|
|
Preferential phosphorylation of focal adhesion kinase tyrosine 861 is critical for mediating an anti-apoptotic response to hyperosmotic stress.
J. Biol. Chem. 282(14) , 10370-9, (2007) The results presented here demonstrate that focal adhesion kinase (FAK) Tyr-861 is the predominant tyrosine phosphorylation site stimulated by hyperosmotic stress in a variety of cell types, including... |
| cytochalasin a from helminthosporium dematioideum |
| (3E,11Z)-16-Benzyl-13-hydroxy-9,15-diméthyl-14-méthylène-6,7,8,9,10,12a,13,14,15,15a,16,17-dodécahydro-2H-oxacyclotétradécino[2,3-d]isoindole-2,5,18-trione |
| MFCD00005935 |
| 20-dehydro-phomin |
| 2H-Oxacyclotetradecino[2,3-d]isoindole-2,5,18-trione, 6,7,8,9,10,12a,13,14,15,15a,16,17-dodecahydro-13-hydroxy-9,15-dimethyl-14-methylene-16-(phenylmethyl)-, (3E,11Z)- |
| (3E,11Z)-16-Benzyl-13-hydroxy-9,15-dimethyl-14-methylene-6,7,8,9,10,12a,13,14,15,15a,16,17-dodecahydro-2H-oxacyclotetradecino[2,3-d]isoindole-2,5,18-trione |
| 7(S)-Hydroxy-16(R)-methyl-10-phenyl-24-oxa(14)cytochalasa-6(12),13(E),21(E)-triene-1,20,23-trione |
| CytochalasinA from Drechslera dematioidea |
| Cytochalasin A |
| (3E,11Z)-16-benzyl-13-hydroxy-9,15-dimethyl-14-methylidene-6,7,8,9,10,12a,13,14,15,15a,16,17-dodecahydro-2H-oxacyclotetradecino[2,3-d]isoindole-2,5,18-trione |
| Dehydrophomin |
| EINECS 237-964-9 |
| Cytochalasin dreschslera dematioidea |
| (3E,11Z)-16-Benzyl-13-hydroxy-9,15-dimethyl-14-methylen-6,7,8,9,10,12a,13,14,15,15a,16,17-dodecahydro-2H-oxacyclotetradecino[2,3-d]isoindol-2,5,18-trion |
| 5,5-Didehydrophomin |