HOE 140 TFA
Modify Date: 2025-08-20 14:31:55
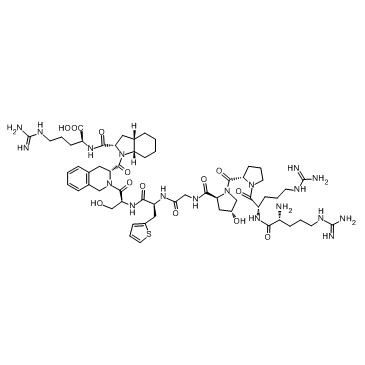
HOE 140 TFA structure
|
Common Name | HOE 140 TFA | ||
|---|---|---|---|---|
| CAS Number | 130308-48-4 | Molecular Weight | 1304.522 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C59H89N19O13S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of HOE 140 TFAIcatibant(HOE-140) is a selective and specific antagonist of bradykinin B2 receptor with IC50 and Ki of 1.07 nM and 0.798 nM respectively.IC50 value: 1.07 nM [1]Target: bradykinin B2 receptor antgonistin vitro: In receptor binding studies in guinea-pig ileum preparations, Hoe 140 showed an IC50 of 1.07 x 10(-9) mol l-1 and a KI value of 7.98 x 10(-10) mol l-1. In isolated organ preparations Hoe 140 and D-Arg-[Hyp2,Thi5,8, D-Phe7]BK inhibited bradykinin-induced contractions concentration dependently, with IC50-values in the guinea-pig ileum preparation of 1.1 x 10(-8) mol l-1 and 3 x 10(-5) mol l-1, respectively. pA2 values in this tissue were 8.42 and 6.18, respectively [1].in vivo: HOE 140 (1, 3, or 10 μg/10 μl physiological saline) was administered into the wound by a sterile micropipette. HOE 140 (1, 3, 10 μg) significantly relieved mechanical allodynia and guarding in comparison with vehicle-treated group [2]. HOE-140 (10 nmol/kg) protected against memory impairment. This treatment attenuated the brain edema, interleukin-1β, tumor necrosis factor-α, and nitric oxide metabolites content elicited by mLFPI. Accordingly, HOE-140 administration protected against the increase of nicotinamide adenine dinucleotide phosphate oxidase activity, thiobarbituric-acid-reactive species, protein carbonylation generation, and Na+ K+ ATPase inhibition induced by trauma [3]. |
| Name | icatibant |
|---|---|
| Synonym | More Synonyms |
| Description | Icatibant(HOE-140) is a selective and specific antagonist of bradykinin B2 receptor with IC50 and Ki of 1.07 nM and 0.798 nM respectively.IC50 value: 1.07 nM [1]Target: bradykinin B2 receptor antgonistin vitro: In receptor binding studies in guinea-pig ileum preparations, Hoe 140 showed an IC50 of 1.07 x 10(-9) mol l-1 and a KI value of 7.98 x 10(-10) mol l-1. In isolated organ preparations Hoe 140 and D-Arg-[Hyp2,Thi5,8, D-Phe7]BK inhibited bradykinin-induced contractions concentration dependently, with IC50-values in the guinea-pig ileum preparation of 1.1 x 10(-8) mol l-1 and 3 x 10(-5) mol l-1, respectively. pA2 values in this tissue were 8.42 and 6.18, respectively [1].in vivo: HOE 140 (1, 3, or 10 μg/10 μl physiological saline) was administered into the wound by a sterile micropipette. HOE 140 (1, 3, 10 μg) significantly relieved mechanical allodynia and guarding in comparison with vehicle-treated group [2]. HOE-140 (10 nmol/kg) protected against memory impairment. This treatment attenuated the brain edema, interleukin-1β, tumor necrosis factor-α, and nitric oxide metabolites content elicited by mLFPI. Accordingly, HOE-140 administration protected against the increase of nicotinamide adenine dinucleotide phosphate oxidase activity, thiobarbituric-acid-reactive species, protein carbonylation generation, and Na+ K+ ATPase inhibition induced by trauma [3]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C59H89N19O13S |
| Molecular Weight | 1304.522 |
| Exact Mass | 1303.660767 |
| PSA | 544.46000 |
| LogP | -2.63 |
| Index of Refraction | 1.742 |
| InChIKey | QURWXBZNHXJZBE-OVZQYVDUSA-N |
| SMILES | NC(N)=NCCCC(N)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(=O)N1CC(O)CC1C(=O)NCC(=O)NC(Cc1cccs1)C(=O)NC(CO)C(=O)N1Cc2ccccc2CC1C(=O)N1C(C(=O)NC(CCCN=C(N)N)C(=O)O)CC2CCCCC21 |
| Storage condition | −20°C |
| WGK Germany | 3 |
|---|
| D-Arginyl-L-arginyl-L-prolyl-trans-4-hydroxy-L-prolylglycyl-3-(2-thienyl)-L-alanyl-L-seryl-D-1,2,3,4-tetrahydro-3-isoquinolinecarbonyl-L-(2a,3ab,7ab)-octahydro-1H-indole-2-carbonyl-L-arginine |
| (2S)-2-({[(3aS,7aS)-1-({(3R)-2-[(2S)-2-{[(2S)-2-{[({[(2S,4R)-1-({(2S)-1-[(2S)-2-{[(2R)-2-Amino-5-carbamimidamidopentanoyl]amino}-5-carbamimidamidopentanoyl]pyrrolidin-2-yl}carbonyl)-4-hydroxypyrrolidin-2-yl]carbonyl}amino)acetyl]amino}-3-(2-thienyl)propanoyl]amino}-3-hydroxypropanoyl]-1,2,3,4-tetrahydroisoquinolin-3-yl}carbonyl)octahydro-1H-indol-2-yl]carbonyl}amino)-5-carbamimidamidopentanoic acid (non-preferred name) |
| H0E-140 |
| HOE I40 |
| (2S)-2-({[(3aS,7aS)-1-({(3R)-2-[(2S)-2-{[(2S)-2-{[({[(2S,4R)-1-({(2S)-1-[(2S)-2-{[(2R)-2-Amino-5-carbamimidamidopentanoyl]amino}-5-carbamimidamidopentanoyl]-2-pyrrolidinyl}carbonyl)-4-hydroxy-2-pyrrolidinyl]carbonyl}amino)acetyl]amino}-3-(2-thienyl)propanoyl]amino}-3-hydroxypropanoyl]-1,2,3,4-tetrahydro-3-isoquinolinyl}carbonyl)octahydro-1H-indol-2-yl]carbonyl}amino)-5-carbamimidamidopentanoic acid |
| Icatibant |