Balofloxacin
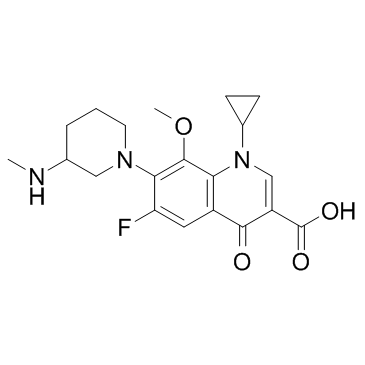
Balofloxacin structure
|
Common Name | Balofloxacin | ||
|---|---|---|---|---|
| CAS Number | 127294-70-6 | Molecular Weight | 389.42 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 608.3±55.0 °C at 760 mmHg | |
| Molecular Formula | C20H24FN3O4 | Melting Point | 137ºC | |
| MSDS | USA | Flash Point | 321.7±31.5 °C | |
Use of BalofloxacinBalofloxacin is quinolone antibiotic, inhibiting the synthesis of bacterial DNA by interference with the enqyme DNA gyrase.Target: Antibacterial; DNA gyrase.Balofloxacin, an orally active fluoroquinolone antibiotic, has been developed by Choongwae Pharma in Korea, for the treatment of urinary tract infection (UTI). Chugai and Ciba were developing balofloxacin for respiratory tract infections (RTI) but discontinued development in 1995 due to changes in Chugai's R&D focus and a lack of efficacy of the drug. Following phase II trials, Choongwae bought the rights to develop balofloxacin in Korea from Chugai. Phase III trials for UTI were completed in early 2001. Balofloxacin was approved by the Korean FDA in December 2001 for UTI. In March 2002, phase II trials were underway for RTI. |
| Name | Balofloxacin |
|---|---|
| Synonym | More Synonyms |
| Description | Balofloxacin is quinolone antibiotic, inhibiting the synthesis of bacterial DNA by interference with the enqyme DNA gyrase.Target: Antibacterial; DNA gyrase.Balofloxacin, an orally active fluoroquinolone antibiotic, has been developed by Choongwae Pharma in Korea, for the treatment of urinary tract infection (UTI). Chugai and Ciba were developing balofloxacin for respiratory tract infections (RTI) but discontinued development in 1995 due to changes in Chugai's R&D focus and a lack of efficacy of the drug. Following phase II trials, Choongwae bought the rights to develop balofloxacin in Korea from Chugai. Phase III trials for UTI were completed in early 2001. Balofloxacin was approved by the Korean FDA in December 2001 for UTI. In March 2002, phase II trials were underway for RTI. |
|---|---|
| Related Catalog | |
| References |
[1]. Alksne L. Balofloxacin Choongwae. Curr Opin Investig Drugs. 2003 Feb;4(2):224-9. |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 608.3±55.0 °C at 760 mmHg |
| Melting Point | 137ºC |
| Molecular Formula | C20H24FN3O4 |
| Molecular Weight | 389.42 |
| Flash Point | 321.7±31.5 °C |
| PSA | 60.69000 |
| LogP | 1.24 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.639 |
| InChIKey | MGQLHRYJBWGORO-UHFFFAOYSA-N |
| SMILES | CNC1CCCN(c2c(F)cc3c(=O)c(C(=O)O)cn(C4CC4)c3c2OC)C1 |
| Storage condition | 2-8°C |
|
Material Safety Data Sheet
Section1. Identification of the substance Product Name: Balofloxacin Synonyms: Section2. Hazards identification Harmful by inhalation, in contact with skin, and if swallowed. Section3. Composition/information on ingredients. Ingredient name:Balofloxacin CAS number:127294-70-6 Section4. First aid measures Skin contact:Immediately wash skin with copious amounts of water for at least 15 minutes while removing contaminated clothing and shoes. If irritation persists, seek medical attention. Eye contact:Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical attention. Inhalation:Remove to fresh air. In severe cases or if symptoms persist, seek medical attention. Ingestion:Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention. Section5. Fire fighting measures In the event of a fire involving this material, alone or in combination with other materials, use dry powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus should be worn. Section6. Accidental release measures Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national standards. Respiratory precaution:Wear approved mask/respirator Hand precaution:Wear suitable gloves/gauntlets Skin protection:Wear suitable protective clothing Eye protection:Wear suitable eye protection Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container for disposal. See section 12. Environmental precautions: Do not allow material to enter drains or water courses. Section7. Handling and storage Handling:This product should be handled only by, or under the close supervision of, those properly qualified in the handling and use of potentially hazardous chemicals, who should take into account the fire, health and chemical hazard data given on this sheet. Store in closed vessels, refrigerated. Storage: Section8. Exposure Controls / Personal protection Engineering Controls: Use only in a chemical fume hood. Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles. General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse. Section9. Physical and chemical properties Appearance:Not specified Boiling point:No data No data Melting point: Flash point:No data Density:No data Molecular formula:C20H24FN3O4 Molecular weight:389.4 Section10. Stability and reactivity Conditions to avoid: Heat, flames and sparks. Materials to avoid: Oxidizing agents. Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen fluoride. Section11. Toxicological information No data. Section12. Ecological information No data. Section13. Disposal consideration Arrange disposal as special waste, by licensed disposal company, in consultation with local waste disposal authority, in accordance with national and regional regulations. Section14. Transportation information Non-harzardous for air and ground transportation. Section15. Regulatory information No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302, or have known CAS numbers that exceed the threshold reporting levels established by SARA Title III, Section 313. SECTION 16 - ADDITIONAL INFORMATION N/A |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Determination of the new fluoroquinolone balofloxacin and its metabolites in biological fluids by high performance liquid chromatography.
Arzneimittelforschung 45(6) , 716-8, (1995) A sensitive high performance liquid chromatographic method for the determination in biological fluids of 1-cyclopropyl-6-fluoro-1,4- dihydro-8-methoxy-7-(3-methylaminopiperidin-1-yl)-4-oxoquinoline-3 ... |
|
|
Photoallergenicity of a fluoroquinolone antibacterial agent with a fluorine substituent at the 8-position in guinea pigs exposed to long-wavelength UV light.
Skin Pharmacol. Appl. Skin Physiol. 11(4-5) , 232-40, (1998) The 8-position of the quinolone ring of balofloxacin (BLFX), one of fluoroquinolones, was replaced with fluorine to obtain the 8-F. When an aqueous solution of bovine serum albumin (BSA) containing th... |
|
|
Gatifloxacin, moxifloxacin, and balofloxacin resistance due to mutations in the gyrA and parC genes of Staphylococcus epidermidis strains isolated from patients with endophthalmitis, corneal ulcers and conjunctivitis.
Ophthalmic Res. 42(1) , 43-8, (2009) Staphylococcus epidermidis is considered a commensal bacterium; however, it is frequently isolated from ocular infections showing a multidrug resistance. Ciprofloxacin-resistant strains have been isol... |
| (±)-1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[3-(methylamino)-1-piperidinyl]-4-oxo-3-quinolinecarboxylic acid |
| q35 |
| MFCD00864925 |
| 3-Quinolinecarboxylic acid, 1-cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[3-(methylamino)-1-piperidinyl]-4-oxo- |
| baloxin |
| 1-Cyclopropyl-6-fluoro-8-methoxy-7-[3-(methylamino)-1-piperidinyl]-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid |
| BALOFLOXACIN EXTRA PURE |
| 1-cyclopropyl-6-fluoro-8-methoxy-7-[3-(methylamino)piperidin-1-yl]-4-oxo-1,4-dihydroquinoline-3-carboxylic acid |
| Balofloxacin |

