Propan-2-one oxime
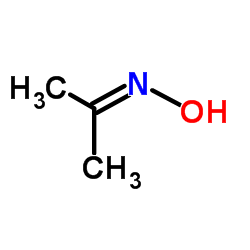
Propan-2-one oxime structure
|
Common Name | Propan-2-one oxime | ||
|---|---|---|---|---|
| CAS Number | 127-06-0 | Molecular Weight | 73.094 | |
| Density | 0.9±0.1 g/cm3 | Boiling Point | 135.0±0.0 °C at 760 mmHg | |
| Molecular Formula | C3H7NO | Melting Point | 60-63 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 45.2±8.0 °C | |
| Name | acetone oxime |
|---|---|
| Synonym | More Synonyms |
| Density | 0.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 135.0±0.0 °C at 760 mmHg |
| Melting Point | 60-63 °C(lit.) |
| Molecular Formula | C3H7NO |
| Molecular Weight | 73.094 |
| Flash Point | 45.2±8.0 °C |
| Exact Mass | 73.052765 |
| PSA | 32.59000 |
| LogP | 0.12 |
| Vapour Pressure | 4.7±0.5 mmHg at 25°C |
| Index of Refraction | 1.410 |
| InChIKey | PXAJQJMDEXJWFB-UHFFFAOYSA-N |
| SMILES | CC(C)=NO |
| Water Solubility | 330 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn:Harmful; |
| Safety Phrases | S22-S24/25 |
| RIDADR | UN1325 |
| WGK Germany | 3 |
| RTECS | AL6825000 |
| Packaging Group | III |
| Hazard Class | 4.1 |
| HS Code | 2928000090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Slow oxidation of acetoxime and methylethyl ketoxime to the corresponding nitronates and hydroxy nitronates by liver microsomes from rats, mice, and humans.
Toxicol. Sci. 47(2) , 144-50, (1999) Acetoxime and methylethyl ketoxime (MEKO) are tumorigenic in rodents, inducing liver tumors in male animals. The mechanisms of tumorigenicity for these compounds are not well defined. Oxidation of the... |
|
|
Synthesis and anticancer evaluation of benzyloxyurea derivatives.
Chem. Pharm. Bull. 62(9) , 898-905, (2014) A series of novel benzyloxyurea derivatives was designed, synthesized by substituting different benzyls or phenyls on N,N'-positions of the hydroxyurea (HU). These target compounds were evaluated for ... |
|
|
Reversion of structure-activity relationships of antitumor platinum complexes by acetoxime but not hydroxylamine ligands.
Mol. Pharmacol. 71(1) , 357-65, (2007) The presence of cis-configured exchangeable ligands has long been considered a prerequisite for antitumor activity of platinum complexes, but over the past few years, several examples violating this s... |
| N-Hydroxypropan-2-imine |
| Acetone, oxime |
| β-Isonitrosopropane |
| MFCD00002118 |
| Acetone oxime |
| 2-Propanone, oxime |
| Acetoxime |
| EINECS 204-820-1 |
| b-Isonitrosopropane |
| N-Hydroxy-2-propanimine |
| N-propan-2-ylidenehydroxylamine |
| propan-2-one oxime |
 CAS#:67-64-1
CAS#:67-64-1 CAS#:7632-00-0
CAS#:7632-00-0 CAS#:7119-91-7
CAS#:7119-91-7 CAS#:79-46-9
CAS#:79-46-9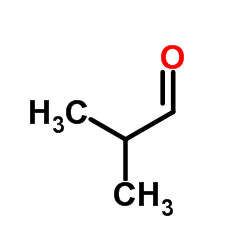 CAS#:78-84-2
CAS#:78-84-2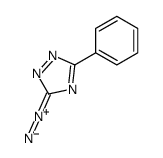 CAS#:80670-36-6
CAS#:80670-36-6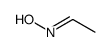 CAS#:5780-37-0
CAS#:5780-37-0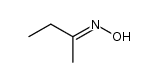 CAS#:10341-63-6
CAS#:10341-63-6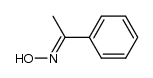 CAS#:10341-75-0
CAS#:10341-75-0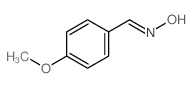 CAS#:3717-21-3
CAS#:3717-21-3![2-Propanone,O-[(methylamino)carbonyl]oxime structure](https://image.chemsrc.com/caspic/325/10520-34-0.png) CAS#:10520-34-0
CAS#:10520-34-0 CAS#:74-89-5
CAS#:74-89-5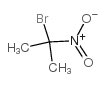 CAS#:5447-97-2
CAS#:5447-97-2![[[(1-methylethylidene)amino]oxy]acetic acid structure](https://image.chemsrc.com/caspic/241/5382-89-8.png) CAS#:5382-89-8
CAS#:5382-89-8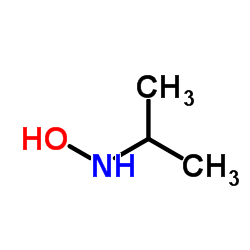 CAS#:5080-22-8
CAS#:5080-22-8 CAS#:5765-61-7
CAS#:5765-61-7 CAS#:1516-60-5
CAS#:1516-60-5 CAS#:100-01-6
CAS#:100-01-6 CAS#:2623-51-0
CAS#:2623-51-0 CAS#:14146-09-9
CAS#:14146-09-9
