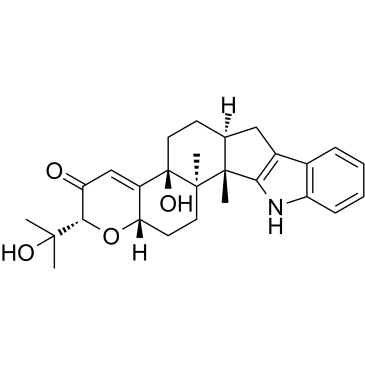
PENITREM A
PENITREM A structure
|
Common Name | PENITREM A | ||
|---|---|---|---|---|
| CAS Number | 12627-35-9 | Molecular Weight | 634.201 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C37H44ClNO6 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Symbol |

GHS06 |
Signal Word | Danger | |
Use of PENITREM APenitrem A is an indole diterpene neurotoxic alkaloid produced by Penicillium, acts as a selective BK channel antagonist with antiproliferative and anti-invasive activities against multiple malignancies. Penitrem A increases the spontaneous release of endogenous glutamate, gamma-aminobutyric acid (GABA) and aspartate from cerebrocortical synaptosomes, and induces tremorgenic syndromes in animals[1][2]. |
| Name | penitrem A |
|---|---|
| Synonym | More Synonyms |
| Description | Penitrem A is an indole diterpene neurotoxic alkaloid produced by Penicillium, acts as a selective BK channel antagonist with antiproliferative and anti-invasive activities against multiple malignancies. Penitrem A increases the spontaneous release of endogenous glutamate, gamma-aminobutyric acid (GABA) and aspartate from cerebrocortical synaptosomes, and induces tremorgenic syndromes in animals[1][2]. |
|---|---|
| Related Catalog | |
| Target |
BK channel[1] |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C37H44ClNO6 |
| Molecular Weight | 634.201 |
| Exact Mass | 633.285706 |
| PSA | 107.47000 |
| LogP | 6.46 |
| Index of Refraction | 1.698 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300-H310-H330 |
| Precautionary Statements | P260-P264-P280-P284-P302 + P350-P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | T+: Very toxic; |
| Risk Phrases | R26/27/28 |
| Safety Phrases | S45;S36/S37/S39 |
| RIDADR | UN 2811 6 |
| RTECS | RY7535000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
|
~% 
PENITREM A CAS#:12627-35-9 |
| Literature: Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), , p. 1539 - 1540 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
|
5-hydroxytryptamine has an endothelium-derived hyperpolarizing factor-like effect on coronary flow in isolated rat hearts.
J. Biomed. Sci. 22 , 42, (2015) 5-hydroxytryptamine (5-HT)-induced coronary artery responses have both vasoconstriction and vasorelaxation components. The vasoconstrictive effects of 5-HT have been well studied while the mechanism(s... |
|
|
Small molecule regulators of autophagy identified by an image-based high-throughput screen.
Proc. Natl. Acad. Sci. U. S. A. 104 , 19023-8, (2007) Autophagy is a lysosome-dependent cellular catabolic mechanism mediating the turnover of intracellular organelles and long-lived proteins. Reduction of autophagy activity has been shown to lead to the... |
|
|
Mechanisms underlying activation of transient BK current in rabbit urethral smooth muscle cells and its modulation by IP3-generating agonists.
Am. J. Physiol. Cell Physiol. 305(6) , C609-22, (2013) We used the perforated patch-clamp technique at 37°C to investigate the mechanisms underlying the activation of a transient large-conductance K(+) (tBK) current in rabbit urethral smooth muscle cells.... |
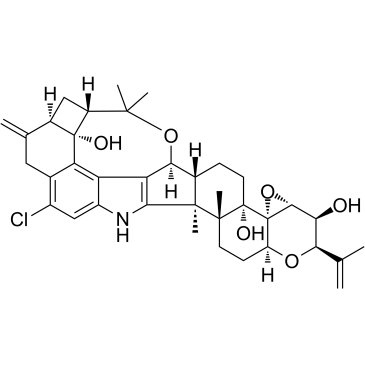
| (2R,3S,3aR,4aS,4bS,6aR,7S,7dR,8S,9aR,14bS,14cR,16aS)-12-chloro-14b,14c,17,17-tetramethyl-10-methylidene-2-(prop-1-en-2-yl)-3,3a,6,6a,7,8,9,9a,10,11,14,14b,14c,15,16,16a-hexadecahydro-2H,4bH-7,8-(epoxymethano)cyclobuta[5,6]benzo[1,2-e]oxireno[4',4a']chromeno[5',6':6,7]indeno[1,2-b]indole-3,4b,7d(5H)-triol |
| (2R,3S,3aR,4aS,4bS,6aR,7S,7dR,8S,9aR,14bS,14cR,16aS)-12-Chloro-2-isopropenyl-14b,14c,17,17-tetramethyl-10-methylene-3,3a,6,6a,7,8,9,9a,10,11,14,14b,14c,15,16,16a-hexadecahydro-2H,4bH-7,8-(epoxymethano)cyclobuta[5,6]benzo[1,2-e]oxireno[4',4a']chromeno[5',6':6,7]indeno[1,2-b]indole-3,4b,7d(5H)-triol |
| MFCD00083465 |
| PENITREM A |
| 4-Methylhistamine dihydrochloride |
| 2H,6H-Benzo[fg]cyclobut[kl]indeno[1,2-d][3]benzoxocin-14,15-imine-3,4b,11c(5H,6aH)-triol, 12-chloro-3,3a,6b,8,8a,9,9a,10,11,15a,15b,16,17,17a-tetradecahydro-8,8,15a,15b-tetramethyl-10-methylene-2-(1-methylethenyl)-, (2R,3S,3aR,4aS,4bS,6aR,6bS,8aS,9aR,11cR,15aS,15bR,17aS)- |
| Tremortin A |
| (2R,3S,3aR,4aS,4bS,6aR,7S,7dR,8S,9aR,14bS,14cR,16aS)-12-Chloro-14b,14c,17,17-tetramethyl-10-methylene-2-(prop-1-en-2-yl)-3,3a,6,6a,7,8,9,9a,10,11,14,14b,14c,15,16,16a-hexadecahydro-2H,4bH-7,8-(epoxymethano)cyclobuta[5,6]benzo[1,2-e]oxireno[4',4a']chromeno[5',6':6,7]indeno[1,2-b]indole-3,4b,7d(5H)-triol |