AM095 (free acid)
Modify Date: 2024-01-02 19:18:25
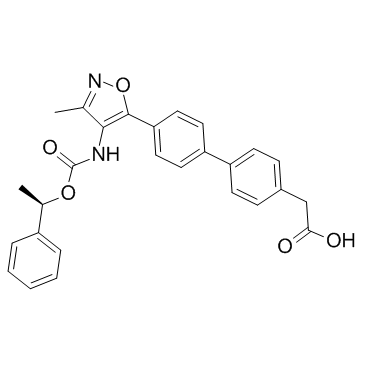
AM095 (free acid) structure
|
Common Name | AM095 (free acid) | ||
|---|---|---|---|---|
| CAS Number | 1228690-36-5 | Molecular Weight | 456.490 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 641.9±55.0 °C at 760 mmHg | |
| Molecular Formula | C27H24N2O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 342.0±31.5 °C | |
Use of AM095 (free acid)AM095 (free acid) is a potent LPA1 receptor antagonist with IC50 values of 0.98 and 0.73 μM for recombinant human or mouse LPA1 respectively. |
| Name | 2-[4-[4-[3-methyl-4-[[(1R)-1-phenylethoxy]carbonylamino]-1,2-oxazol-5-yl]phenyl]phenyl]acetic acid |
|---|---|
| Synonym | More Synonyms |
| Description | AM095 (free acid) is a potent LPA1 receptor antagonist with IC50 values of 0.98 and 0.73 μM for recombinant human or mouse LPA1 respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.98 μM (human LPA1), 0.73 μM (mouse LPA1) |
| In Vitro | AM095 inhibits the LPA-induced calcium flux of CHO cells stably transfected with human or mouse LPA1. The IC50 for AM095 antagonism of LPA-induced calcium flux of human or mouse LPA1-transfected CHO cells is 0.025 and 0.023 μM, respectively[1]. AM095 reduces LPA-induced vasorelaxation by appr 90% at 10 μM as compared to vehicle control[2]. AM095 inhibits LPA-driven chemotaxis of CHO cells overexpressing mouse LPA1 (IC50=778 nM) and human A2058 melanoma cells (IC50=233 nM)[3]. |
| In Vivo | Pharmacological antagonism of LPA1 with AM095 significantly attenuates bleomycin-induced dermal fibrosis[1]. AM095 has high oral bioavailability and a moderate half-life and is well tolerated at the doses tested in rats and dogs after oral and intravenous dosing. AM095 dose-dependently reduces LPA-stimulated histamine release. AM095 attenuates bleomycin-induced increases in collagen, protein, and inflammatory cell infiltration in bronchalveolar lavage fluid. AM095 decreases kidney fibrosis in a mouse unilateral ureteral obstruction model[3]. |
| Kinase Assay | Assays are conducted using both hLPA1/CHO and mLPA1/CHO cells. A cell pellet of hLPA1/CHO or mLPA1/CHO cells is resuspended in appr 20 mL of ice-cold membrane buffer containing 10 mM HEPES, pH 7.4, 1 mM dithiothreitol, and protease inhibitors. Cells are sonicated, and the cell lysate is centrifuged at 2000 rpm for 10 min at 4°C. The supernatant is further centrifuged at 25,000 rpm for 70 min at 4°C. The membrane pellet is resuspended in 5 mL of ice-cold membrane buffer and homogenized using a Potter-Elvehjem tissue grinder. Final protein concentration is determined using the Bradford Protein Assay Kit. Known amounts of AM095 (diluted in dimethyl sulfoxide) or vehicle (dimethyl sulfoxide) are added to 25 to 40 μg of hLPA1/CHO or mLPA1/CHO membranes and 0.1 nM [35S]-GTPγS in buffer (50 mM HEPES, 0.1 mM NaCl, 10 mM MgCl2, 50 μg/mL saponin, pH 7.5) containing 0.2% fatty acid-free human serum albumin and 5 μM GDP. To test for LPA1 antagonist activity, the ability of AM095 to inhibit GTPγS binding stimulated by 900 nM LPA (18:1) is measured. Alternatively, to test for agonist effects, the ability of AM095 to stimulate GTPγS binding in the absence of LPA is measured. Reactions are incubated for 30 min at 30°C, before harvesting membranes onto glass filter binding plates and washing three times with cold buffer containing 50 mM HEPES, pH 7.4, 100 mM NaCl, 10 mM MgCl2 using a Brandel 96-tip cell harvester. Plates are dried and then cpm are evaluated by using a Packard TopCount NXT microplate scintillation counter. |
| Animal Admin | Mice underwent UUO or sham surgery to the left kidney. In brief, a longitudinal, upper left incision is performed to expose the left kidney. The renal artery is located and a 6/0 silk thread is passed between the artery and the ureter. The thread is looped around the ureter and knotted three times insuring full ligation of ureter. The kidney is returned to abdomen, the abdominal muscle is sutured, and the skin is closed with staples. The contralateral (right) kidney served as an uninjured control. AM095 (30 mg/kg) or vehicle (water) is given 1 to 4 h before UUO and then b.i.d. thereafter by oral gavage. After 8 days, mice are euthanized using inhaled CO2, and the kidneys are harvested and cut in half for histopathological and biochemical analysis of fibrosis. To assess fibrosis, half of the kidney is fixed in 10% neutral buffered formalin and stained using Masson's trichrome. The other half of the kidney is frozen at −80°C for subsequent biochemical analysis of collagen content. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 641.9±55.0 °C at 760 mmHg |
| Molecular Formula | C27H24N2O5 |
| Molecular Weight | 456.490 |
| Flash Point | 342.0±31.5 °C |
| Exact Mass | 456.168518 |
| PSA | 105.15000 |
| LogP | 4.94 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.629 |
| Storage condition | -20℃ |
|
~% 
AM095 (free acid) CAS#:1228690-36-5 |
| Literature: AMIDRA PHARMACEUTICALS, INC.; BRITTAIN, Jason, Edward; SEIDERS, Thomas, Jon; KING, Christopher, David Patent: WO2011/159550 A2, 2011 ; Location in patent: Page/Page column 80 ; WO 2011/159550 A2 |
|
~% 
AM095 (free acid) CAS#:1228690-36-5 |
| Literature: AMIDRA PHARMACEUTICALS, INC.; BRITTAIN, Jason, Edward; SEIDERS, Thomas, Jon; KING, Christopher, David Patent: WO2011/159550 A2, 2011 ; WO 2011/159550 A2 |
|
~% 
AM095 (free acid) CAS#:1228690-36-5 |
| Literature: AMIDRA PHARMACEUTICALS, INC.; BRITTAIN, Jason, Edward; SEIDERS, Thomas, Jon; KING, Christopher, David Patent: WO2011/159550 A2, 2011 ; WO 2011/159550 A2 |
|
~% 
AM095 (free acid) CAS#:1228690-36-5 |
| Literature: AMIDRA PHARMACEUTICALS, INC.; BRITTAIN, Jason, Edward; SEIDERS, Thomas, Jon; KING, Christopher, David Patent: WO2011/159550 A2, 2011 ; WO 2011/159550 A2 |
|
~% 
AM095 (free acid) CAS#:1228690-36-5 |
| Literature: AMIDRA PHARMACEUTICALS, INC.; BRITTAIN, Jason, Edward; SEIDERS, Thomas, Jon; KING, Christopher, David Patent: WO2011/159550 A2, 2011 ; WO 2011/159550 A2 |
|
~% 
AM095 (free acid) CAS#:1228690-36-5 |
| Literature: AMIDRA PHARMACEUTICALS, INC.; BRITTAIN, Jason, Edward; SEIDERS, Thomas, Jon; KING, Christopher, David Patent: WO2011/159550 A2, 2011 ; WO 2011/159550 A2 |
| CS-1129 |
| AM-095 free base||AM095 |
| {4'-[3-Methyl-4-({[(1R)-1-phenylethoxy]carbonyl}amino)-1,2-oxazol-5-yl]-4-biphenylyl}acetic acid |
| AM095 (free acid) |
| [1,1'-Biphenyl]-4-acetic acid, 4'-[3-methyl-4-[[[(1R)-1-phenylethoxy]carbonyl]amino]-5-isoxazolyl]- |
| AM-095 free base |
| (R)-2-(4'-(3-methyl-4-((1-phenylethoxy)carbonylamino)isoxazol-5-yl)biphenyl-4-yl)acetic acid |
| AM095 |
| [1,1'-Biphenyl]-4acetic acid,4'-[3-methyl-4-[[[(1R)-1-phenylethoxy]carbonyl]amino]-5-isoxazolyl] |
| {4'-[3-Methyl-4-((R)-1-phenyl-ethoxycarbonylamino)-isoxazol-5-yl]-biphenyl-4-yl}-acetic acid |
![[4’-[3-Methyl-4-[[[((R)-1-Phenylethyl)Oxy]Carbonyl]Amino]Isoxazol-5-Yl]Biphenyl-4-Yl]Acetic Acid Ethyl Ester structure](https://www.chemsrc.com/caspic/066/1228690-38-7.png)


 CAS#:1345614-59-6
CAS#:1345614-59-6