ATB-346
Modify Date: 2025-08-24 21:59:17
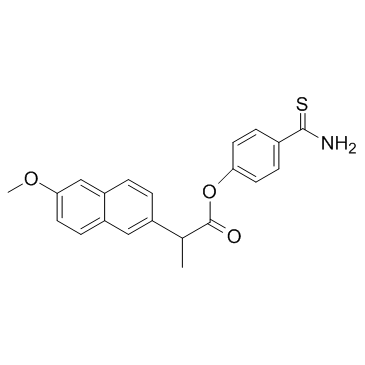
ATB-346 structure
|
Common Name | ATB-346 | ||
|---|---|---|---|---|
| CAS Number | 1226895-20-0 | Molecular Weight | 365.445 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 561.4±60.0 °C at 760 mmHg | |
| Molecular Formula | C21H19NO3S | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 293.3±32.9 °C | |
Use of ATB-346ATB-346 is a novel hydrogen sulphide-releasing derivative of naproxen with markedly reduced toxicity.IC50 value:Target: COX-2 ATB-346 suppressed gastric prostaglandin E(2) synthesis as effectively as naproxen, but produced negligible damage in the stomach and intestine, Unlike naproxen and celecoxib, ATB-346 accelerated healing of pre-existing gastric ulcers. In a mouse airpouch model, ATB-346 suppressed cyclooxygenase-2 activity and inhibited leukocyte infiltration more effectively than naproxen. ATB-346 was as effective as naproxen in adjuvant-induced arthritis in rats, with a more rapid onset of activity. Unlike naproxen, ATB-346 did not elevate blood pressure in hypertensive rats [1]. Treatement with ATB-346 exhibited a significantly more rapid and sustained recovery of motor function, achieving greater than double the increase in locomotion score of the naproxen group by the 10th day of treatment. ATB-346 also significantly reduced the severity of inflammation (proinflammatory cytokines, apoptosis of neural tissue, and nitrosative stress) that characterized the secondary effects of SCI [2]. |
| Name | (4-carbamothioylphenyl) 2-(6-methoxynaphthalen-2-yl)propanoate |
|---|---|
| Synonym | More Synonyms |
| Description | ATB-346 is a novel hydrogen sulphide-releasing derivative of naproxen with markedly reduced toxicity.IC50 value:Target: COX-2 ATB-346 suppressed gastric prostaglandin E(2) synthesis as effectively as naproxen, but produced negligible damage in the stomach and intestine, Unlike naproxen and celecoxib, ATB-346 accelerated healing of pre-existing gastric ulcers. In a mouse airpouch model, ATB-346 suppressed cyclooxygenase-2 activity and inhibited leukocyte infiltration more effectively than naproxen. ATB-346 was as effective as naproxen in adjuvant-induced arthritis in rats, with a more rapid onset of activity. Unlike naproxen, ATB-346 did not elevate blood pressure in hypertensive rats [1]. Treatement with ATB-346 exhibited a significantly more rapid and sustained recovery of motor function, achieving greater than double the increase in locomotion score of the naproxen group by the 10th day of treatment. ATB-346 also significantly reduced the severity of inflammation (proinflammatory cytokines, apoptosis of neural tissue, and nitrosative stress) that characterized the secondary effects of SCI [2]. |
|---|---|
| Related Catalog | |
| Target |
COX |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 561.4±60.0 °C at 760 mmHg |
| Molecular Formula | C21H19NO3S |
| Molecular Weight | 365.445 |
| Flash Point | 293.3±32.9 °C |
| Exact Mass | 365.108551 |
| PSA | 93.64000 |
| LogP | 4.32 |
| Appearance of Characters | light yellow solid |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Storage condition | -20℃ |
| 4-Carbamothioylphenyl 2-(6-methoxy-2-naphthyl)propanoate |
| UNII:3096O7WP53 |
| unii-3096o7wp53 |
| 2-Naphthaleneacetic acid, 6-methoxy-α-methyl-, 4-(aminothioxomethyl)phenyl ester |
| atb-346 |
| ABT-346 |