N-Hydroxy-N'-(4-iodophenyl)octanediamide
Modify Date: 2024-01-11 10:46:46
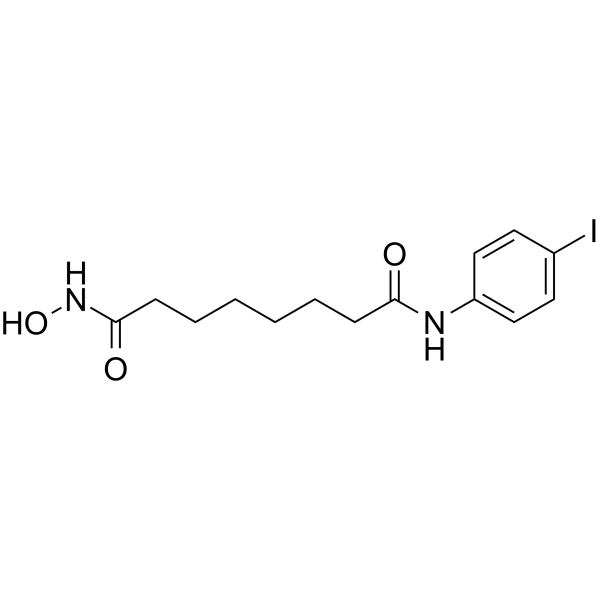
N-Hydroxy-N'-(4-iodophenyl)octanediamide structure
|
Common Name | N-Hydroxy-N'-(4-iodophenyl)octanediamide | ||
|---|---|---|---|---|
| CAS Number | 1219807-87-0 | Molecular Weight | 390.217 | |
| Density | 1.6±0.1 g/cm3 | Boiling Point | N/A | |
| Molecular Formula | C14H19IN2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of N-Hydroxy-N'-(4-iodophenyl)octanediamide4-Iodo-SAHA (1k) is an orally active class I and class II histone deacetylase (HDAC) inhibitor with EC50s of 1.1, 0.95, 0.12, 0.24, 0.85 and 1.3 μM for Skbr3, HT29, U937, JA16 and HL60 cell lines, respectively. 4-Iodo-SAHA (1k) can be used for the research of cancer[1]. |
| Name | N'-hydroxy-N-(4-iodophenyl)octanediamide |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Iodo-SAHA (1k) is an orally active class I and class II histone deacetylase (HDAC) inhibitor with EC50s of 1.1, 0.95, 0.12, 0.24, 0.85 and 1.3 μM for Skbr3, HT29, U937, JA16 and HL60 cell lines, respectively. 4-Iodo-SAHA (1k) can be used for the research of cancer[1]. |
|---|---|
| Related Catalog | |
| In Vitro | 4-Iodo-SAHA (1k) (0.1-100 μM; 48 h ) inhibits Skbr3, HT29, U937, JA16 and HL60 cell lines [1]. 4-Iodo-SAHA (1k) (2 μM; 6-24 h ) affects acetylated H4 and p21 levels in SKBR3 cells[1]. Cell Proliferation Assay[1] Cell Line: SKBR3, HT29, U937, JA16 and HL60 cell lines Concentration: 0.1-100 μM Incubation Time: 48 h Result: Inhibited SKBR3, HT29, U937, JA16 and HL60 cell lines with EC50s of 1.1, 0.95, 0.12, 0.24, 0.85 and 1.3 μM , respectively. Showed 10-fold potent as an inhibitor of U937 cell line compared to SAHA. Western Blot Analysis[1] Cell Line: SKBR3-breast-derived cell line Concentration: 2 μM Incubation Time: 6, 12 and 24 h Result: Time-dependently up regulated histone H4 acetylation and p21/WAF1 cell cycle inhibitor accumulation in SKBR3 cells. |
| In Vivo | 4-Iodo-SAHA (1k) (50 mg/kg; p.o. five times a week for two weeks) compares to SAHA-treated and control mice with similar toxicity[1]. Animal Model: 8-week-old fvb mice[1] Dosage: 50 mg/kg Administration: Oral gavage; 50 mg/kg five times per week; for 2 weeks Result: Compared to both SAHA-treated and control mice with similar body weights and hematological counts. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C14H19IN2O3 |
| Molecular Weight | 390.217 |
| Exact Mass | 390.044037 |
| PSA | 78.43000 |
| LogP | 2.49 |
| Index of Refraction | 1.616 |
| 4-iodo-SAHA |
| Octanediamide, N-hydroxy-N-(4-iodophenyl)- |
| N-Hydroxy-N'-(4-iodophenyl)octanediamide |
| 4-Iodo Suberoylanilide Hydroxamic Acid |