Trimetazidine-d8 dihydrochloride
Modify Date: 2025-08-27 12:33:48
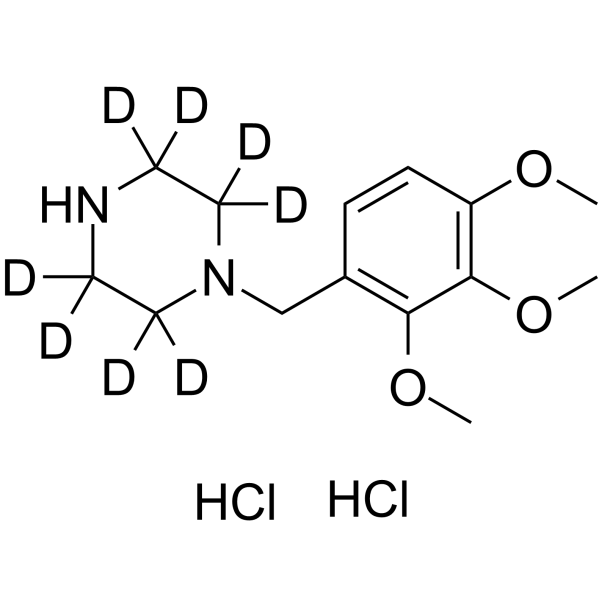
Trimetazidine-d8 dihydrochloride structure
|
Common Name | Trimetazidine-d8 dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 1219795-37-5 | Molecular Weight | 347.31 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C14H16D8Cl2N2O3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Trimetazidine-d8 dihydrochlorideTrimetazidine-d8 dihydrochloride is the deuterium labeled Trimetazidine dihydrochloride. Trimetazidine dihydrochloride is a selective long chain 3-ketoyl coenzyme A thiolase inhibitor with an IC50 of 75 nM, which can inhibit β-oxidation of free fatty acid (FFA). Trimetazidine dihydrochloride is an effective antianginal agent and a cytoprotective drug, has anti-oxidant, anti-inflammatory, antinociceptive and gastroprotective properties. Trimetazidine dihydrochloride triggers autophagy. Trimetazidine dihydrochloride is also a 3-hydroxyacyl-CoA dehydrogenase (HADHA) inhibitor[1][2][3][4]. |
| Name | Trimetazidine-d8 dihydrochloride |
|---|
| Description | Trimetazidine-d8 dihydrochloride is the deuterium labeled Trimetazidine dihydrochloride. Trimetazidine dihydrochloride is a selective long chain 3-ketoyl coenzyme A thiolase inhibitor with an IC50 of 75 nM, which can inhibit β-oxidation of free fatty acid (FFA). Trimetazidine dihydrochloride is an effective antianginal agent and a cytoprotective drug, has anti-oxidant, anti-inflammatory, antinociceptive and gastroprotective properties. Trimetazidine dihydrochloride triggers autophagy. Trimetazidine dihydrochloride is also a 3-hydroxyacyl-CoA dehydrogenase (HADHA) inhibitor[1][2][3][4]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C14H16D8Cl2N2O3 |
|---|---|
| Molecular Weight | 347.31 |
| InChIKey | VYFLPFGUVGMBEP-IKGFOUCPSA-N |
| SMILES | COc1ccc(CN2CCNCC2)c(OC)c1OC.Cl.Cl |
| Hazard Codes | Xi |
|---|