Anastrozole
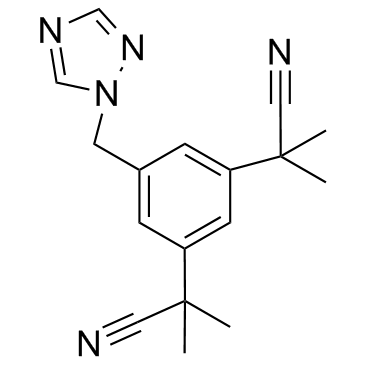
Anastrozole structure
|
Common Name | Anastrozole | ||
|---|---|---|---|---|
| CAS Number | 120511-73-1 | Molecular Weight | 293.366 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 469.7±55.0 °C at 760 mmHg | |
| Molecular Formula | C17H19N5 | Melting Point | 81-82°C | |
| MSDS | Chinese USA | Flash Point | 237.9±31.5 °C | |
| Symbol |


GHS07, GHS08 |
Signal Word | Danger | |
Use of AnastrozoleAnastrozole is a potent, highly selective aromatase inhibitor, which inhibits human placental aromatase with an IC50 of 15 nM. |
| Name | anastrozole |
|---|---|
| Synonym | More Synonyms |
| Description | Anastrozole is a potent, highly selective aromatase inhibitor, which inhibits human placental aromatase with an IC50 of 15 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 15 nM (human aromatase)[1] |
| In Vitro | Anastrozole is a comparatively simple, achiral benzyltriazole derivative, that inhibits human placental aromatase with an IC50 of 15 nM. In the same assay it is 200 times as potent as aminoglutethimide (AG), twice as potent as 4-OHA and one third as potent as Fadrozole[1]. |
| In Vivo | Groups of eight immature (22-day-old) female rats are given androstenedione (AD) (30 mg/kg) in arachis oil s.c. daily for3 days with or without various doses of Anastrozole p.o. on day 4 the uteri are dissected, blotted and weighed. An oral dose of 0.1 mg/kg of Anastrozole given on day 2 or day 3 of the cycle completely blocked ovulation. At the same daily dosage (0.1 mg/kg), Anastrozole completely extinguished the uterotrophic activity of exogenous AD in immature rats. In male pigtailed monkeys, twice-daily oral treatment with 0.1 mg/kg and above of Anastrozole reduced circulating oestradiol concentrations by 50-60%[1]. |
| Kinase Assay | Aromatase inhibition is measured using human placental microsomes and the method of Thompson and Siiteri with Testosterone (0.5 μM) as substrate. 11-hydroxylase inhibition is determined by measuring the conversion of [1,2,6,7-3H]-ll-deoxy- cortisol to cortisol using freshly prepared mitochondria from guinea pig, dog and cow adrenal glands. Reaction products areextracted into chloroform and separated by thin layer chromatography[1]. |
| Animal Admin | Mice[1] Groups of at least eight adult female rats, housed in controlled lighting (on 06.00-20.00 h) and temperature (24±2°C) and undergoing 4-day oestrous cycles, are treated p.o. with a single dose of Anastrozole (0.01-0.1 mg/kg), Fadrozole (0.01-0.1 mg/kg) or AG (5-20 mg/kg) on day 2 at 16.00 h or day 3 at 12.00 h. The presence or absence of eggs in the oviducts on day 1 of the next cycle is then determined. Ovulation is considered blocked when no eggs are found. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 469.7±55.0 °C at 760 mmHg |
| Melting Point | 81-82°C |
| Molecular Formula | C17H19N5 |
| Molecular Weight | 293.366 |
| Flash Point | 237.9±31.5 °C |
| Exact Mass | 293.164032 |
| PSA | 78.29000 |
| LogP | 0.97 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.580 |
| InChIKey | YBBLVLTVTVSKRW-UHFFFAOYSA-N |
| SMILES | CC(C)(C#N)c1cc(Cn2cncn2)cc(C(C)(C)C#N)c1 |
| Storage condition | Store at RT |
| Symbol |


GHS07, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H302-H360 |
| Precautionary Statements | P201-P280-P301 + P312 + P330-P308 + P313 |
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-37/39 |
| RIDADR | 3249 |
| RTECS | CZ1465000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Changes in bone mineral density at 3 years in postmenopausal women receiving anastrozole and risedronate in the IBIS-II bone substudy: an international, double-blind, randomised, placebo-controlled trial.
Lancet Oncol. 15(13) , 1460-8, (2014) Aromatase inhibitors prevent breast cancer in postmenopausal women at high risk of the disease but are associated with accelerated bone loss. We assessed effectiveness of oral risedronate for preventi... |
|
|
NSAID use reduces breast cancer recurrence in overweight and obese women: role of prostaglandin-aromatase interactions.
Cancer Res. 74(16) , 4446-57, (2014) Obesity is associated with a worse breast cancer prognosis and elevated levels of inflammation, including greater cyclooxygenase-2 (COX-2) expression and activity in adipose-infiltrating macrophages. ... |
|
|
Impact of BMI on serum estradiol and bone turnover markers in postmenopausal women with hormone-sensitive early breast cancer treated with anastrozole.
J. Cancer Res. Clin. Oncol. 140(1) , 159-66, (2014) Obesity increases the risk of all-cause and breast cancer mortality. As obese patients have higher levels of aromatase enzyme activity, conflicting results on the effect of body mass index (BMI) of a ... |
| Anastrozole |
| ICI-D-1033 |
| ZD-1033 |
| 2,2'-5-(1H-1,2,4-triazol-1-ylmethyl)benzene-1,3-diylbis(2-methylpropanenitrile) |
| Anastrol |
| MFCD00866298 |
| 1,3-Benzenediacetonitrile, α,α,α',α'-tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)- |
| 2,2'-[5-(1H-1,2,4-Triazol-1-ylmethyl)-1,3-phenylene]bis(2-methylpropanenitrile) |
| ICI D 1033 |
| 1,3-benzenediacetonitrile |
| 2,2'-[5-(1H-1,2,4-triazol-1-ylméthyl)benzène-1,3-diyl]bis(2-méthylpropanenitrile) |
| 2,2'-[5-(1H-1,2,4-Triazol-1-ylmethyl)benzol-1,3-diyl]bis(2-methylpropanonitril) |
| ANASTRAZOLE |
| 2,2'-(5-((1H-1,2,4-Triazol-1-yl)methyl)-1,3-phenylene)bis(2-methylpropanenitrile) |
| 4-Triazol-1-ylmethyl)-1 |
| Anastrolozole |
| Anatrozole |
| a,a,a',a'-Tetramethyl-5-(1H-,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile |
| Anastrozol |
| ARIMIDEX |
 CAS#:288-88-0
CAS#:288-88-0 CAS#:120511-84-4
CAS#:120511-84-4 CAS#:41253-21-8
CAS#:41253-21-8 CAS#:120511-72-0
CAS#:120511-72-0 CAS#:1015083-81-4
CAS#:1015083-81-4![2-[3-(chloromethyl)-5-(2-cyanopropan-2-yl)phenyl]-2-methylpropanenitrile Structure](https://image.chemsrc.com/caspic/281/120511-91-3.png) CAS#:120511-91-3
CAS#:120511-91-3![2,2'-[5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-phenylene]-di(2-methylpropionitrile) hydrobromide Structure](https://image.chemsrc.com/caspic/159/876514-43-1.png) CAS#:876514-43-1
CAS#:876514-43-1 CAS#:120511-88-8
CAS#:120511-88-8 CAS#:13578-51-3
CAS#:13578-51-3
