Limonin

Limonin structure
|
Common Name | Limonin | ||
|---|---|---|---|---|
| CAS Number | 1180-71-8 | Molecular Weight | 470.512 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 668.4±55.0 °C at 760 mmHg | |
| Molecular Formula | C26H30O8 | Melting Point | 298ºC | |
| MSDS | Chinese USA | Flash Point | 358.0±31.5 °C | |
Use of LimoninLimonin is a triterpenoid enriched in citrus fruits, which has antivirus and antitumor ability.IC50 Value: Target: HIV; anticancerLimonin is a triterpenoid aglycone that is a bitter principle of citrus fruits. Limonin is chemically induced carcinogenesis inhibitor and HIV-1 replication inhibitor. Limonin has anti-proliferative, proapoptotic activity on several cancer cell lines and inhibits azoxymethane-induced colon cancer in rats. Limonin also inhibits HIV-1 replication in culturedf monocytes, macrophages, and mononuclear cells, perhaps by inhibition of HIV-1 protease activity. |
| Name | limonin |
|---|---|
| Synonym | More Synonyms |
| Description | Limonin is a triterpenoid enriched in citrus fruits, which has antivirus and antitumor ability.IC50 Value: Target: HIV; anticancerLimonin is a triterpenoid aglycone that is a bitter principle of citrus fruits. Limonin is chemically induced carcinogenesis inhibitor and HIV-1 replication inhibitor. Limonin has anti-proliferative, proapoptotic activity on several cancer cell lines and inhibits azoxymethane-induced colon cancer in rats. Limonin also inhibits HIV-1 replication in culturedf monocytes, macrophages, and mononuclear cells, perhaps by inhibition of HIV-1 protease activity. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 668.4±55.0 °C at 760 mmHg |
| Melting Point | 298ºC |
| Molecular Formula | C26H30O8 |
| Molecular Weight | 470.512 |
| Flash Point | 358.0±31.5 °C |
| Exact Mass | 470.194061 |
| PSA | 104.57000 |
| LogP | 1.66 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.602 |
| InChIKey | KBDSLGBFQAGHBE-MSGMIQHVSA-N |
| SMILES | CC1(C)OC2CC(=O)OCC23C1CC(=O)C1(C)C3CCC2(C)C(c3ccoc3)OC(=O)C3OC321 |
| Hazard Codes | Xn: Harmful; |
|---|---|
| Risk Phrases | R20/21/22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | RR1122000 |
|
Grapefruit (Citrus paradisi Macfad) phytochemicals composition is modulated by household processing techniques.
J. Food Sci. 77(9) , C921-6, (2012) Grapefruits (Citrus paradisi Macfad) contain several phytochemicals known to have health maintaining properties. Due to the consumer's interest in obtaining high levels of these phytochemicals, it is ... |
|
|
Ethylene degreening modulates health promoting phytochemicals in Rio Red grapefruit.
Food Chem. 188 , 77-83, (2015) In the current study, we examined the effects of postharvest degreening and storage on phytochemicals in Rio Red grapefruit. Grapefruits were degreened with 3.5 μl/l of ethylene at 21 °C and 80% relat... |
|
|
Quinine Bitterness and Grapefruit Liking Associate with Allelic Variants in TAS2R31.
Chem. Senses 40 , 437-43, (2015) Multiple psychophysical gene-association studies suggest a single nucleotide polymorphism (SNP) within the bitter receptor gene TAS2R19 on chromosome 12 may be functional. Previously, the Arg299Cys SN... |
| Limonoic acid di-δ-lactone |
| 8-(3-Furyl)decahydro-2,2,4a,8a-tetramethyl-11H,13H-oxireno[d]pyrano[4',3':3,3a]isobenzofuro[5,4-f][2]benzopyran-4,6,13(2H,5aH)-trione |
| (4aS,6aR,8aR,8bR,9aS,12S,12aS,14aR,14bR)-12-(Furan-3-yl)-6,6,8a,12a-tetramethyldecahydrooxireno[2,3-d]pyrano[4',3':3,3a]isobenzofuro[5,4-f]isochromene-3,8,10(1H,6H,8aH)-trione |
| Limonoic acid 3,19:16,17 dilactone |
| 1H,3H-Oxireno[c]pyrano[4'',3'':2',3']furo[3',4':5,6]naphtho[1,2-d]pyran-3,8,10(6H,9aH)-trione, 12-(3-furanyl)decahydro-6,6,8a,12a-tetramethyl-, (4aS,6aR,8aR,8bR,9aS,12S,12aS,14aR,14bR)- |
| LiMone |
| DICTAMNOLACTONE |
| CITROLIMONIN |
| Limonin |
| Evodine |
| Limonoic Acid Di-d-lactone |
| LIMONINE |
| (4aS,6aR,8aR,8bR,9aS,12R,12aS,14aR,14bR)-12-(furan-3-yl)-6,6,8a,12a-tetramethyldecahydro-3H-oxireno[d]pyrano[4',3':3,3a][2]benzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
| EVODIN |
| OBACULACTONE |
| (4aS,6aR,8aR,8bR,9aS,12S,12aS,14aR,14bR)-12-(3-Furyl)-6,6,8a,12a-tetramethyldecahydro-3H-oxireno[d]pyrano[4',3':3,3a][2]benzofuro[5,4-f]isochromene-3,8,10(6H,9aH)-trione |
![11H,13H-Oxireno[d]pyrano[4',3':3,3a]isobenzofuro[5,4-f][2]benzopyran-4,6,13(2H,5aH)-trione, decahydro-2,2,4a,8a-tetramethyl-8-(tetrahydro-3-furanyl)-, (2aR,4aR,4bR,5aS,8S,8aS,10aR,10bR,14aS)- (9CI) structure](https://image.chemsrc.com/caspic/471/1062-07-3.png) CAS#:1062-07-3
CAS#:1062-07-3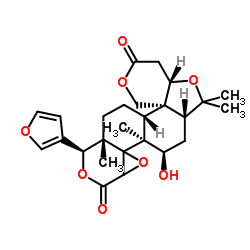 CAS#:989-61-7
CAS#:989-61-7![11H,13H-Oxireno[d]pyrano[4',3':3,3a]isobenzofuro[5,4-f][2]benzopyran-6,13(5aH)-dione,8-(3-furanyl)dodecahydro-4-hydroxy-2,2,4a,8a-tetramethyl-,(2aR,4S,4aS,4bR,5aS,8S,8aS,10aR,10bR,14aS)- (9CI) structure](https://image.chemsrc.com/caspic/436/1258-86-2.png) CAS#:1258-86-2
CAS#:1258-86-2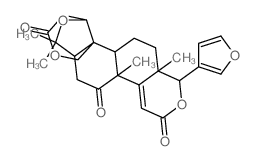 CAS#:989-23-1
CAS#:989-23-1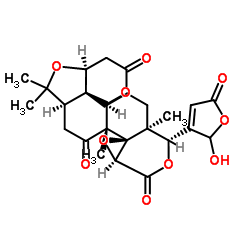 CAS#:99026-99-0
CAS#:99026-99-0
