Trihydrido(pyridine)boron
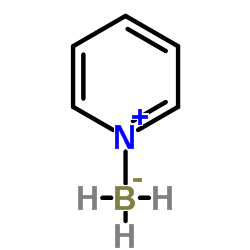
Trihydrido(pyridine)boron structure
|
Common Name | Trihydrido(pyridine)boron | ||
|---|---|---|---|---|
| CAS Number | 110-51-0 | Molecular Weight | 92.935 | |
| Density | 0.929 g/mL at 20 °C | Boiling Point | 102 °C (0.37505 mmHg) | |
| Molecular Formula | C5H8BN | Melting Point | 10-11 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 70 °F | |
| Symbol |


GHS02, GHS06 |
Signal Word | Danger | |
| Name | Borane-pyridine complex |
|---|---|
| Synonym | More Synonyms |
| Density | 0.929 g/mL at 20 °C |
|---|---|
| Boiling Point | 102 °C (0.37505 mmHg) |
| Melting Point | 10-11 °C(lit.) |
| Molecular Formula | C5H8BN |
| Molecular Weight | 92.935 |
| Flash Point | 70 °F |
| Exact Mass | 93.074982 |
| PSA | 4.93000 |
| Vapour Pressure | 22.8mmHg at 25°C |
| Index of Refraction | n20/D 1.532(lit.) |
| Storage condition | 2-8°C |
| Water Solubility | reacts slowly |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS02, GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301-H310 + H330-H315-H319 |
| Precautionary Statements | P210-P260-P280-P284-P301 + P310-P302 + P350 |
| Hazard Codes | F:Flammable;T+:Verytoxic; |
| Risk Phrases | R10;R15;R20;R24/25;R26;R36/37/38;R44 |
| Safety Phrases | S26-S36/37-S43-S45-S36/37/39-S28A |
| RIDADR | UN 1992 3/PG 2 |
| WGK Germany | 2 |
| RTECS | US3675000 |
| Packaging Group | II |
| Hazard Class | 4.3 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
|
Dendritic Glycopolymer as Drug Delivery System for Proteasome Inhibitor Bortezomib in a Calcium Phosphate Bone Cement: First Steps Toward a Local Therapy of Osteolytic Bone Lesions.
Macromol. Biosci. 15 , 1283-95, (2015) Establishment of drug delivery system (DDS) in bone substitute materials for local treatment of bone defects still requires ambitious solutions for a retarded drug release. We present two novel DDS, a... |
|
|
Hydroboration with pyridine borane at room temperature.
J. Am. Chem. Soc. 127 , 5766-5767, (2005) Treatment of pyridine borane (Py.BH3) with iodine, bromine, or strong acids affords activated Py.BH2X complexes that are capable of hydroborating alkenes at room temperature. Evidence is presented for... |
|
|
Global amine and acid functional group modification of proteins.
Anal. Chem. 80(3) , 713-20, (2008) A sequential reaction methodology is employed for the complete derivatization of protein thiols, amines, and acids in high purity under denaturing conditions. Following standard thiol alkylation, prot... |
| Pyridine Borane |
| Borane pyridine complex |
| boron,pyridine |
| Boron, trihydro(pyridine)- |
| EINECS 203-773-4 |
| Trihydrido(pyridine)boron |
| MFCD00012435 |
| Borane - Pyridine CoMplex |
 CAS#:110-86-1
CAS#:110-86-1 CAS#:16940-66-2
CAS#:16940-66-2 CAS#:628-13-7
CAS#:628-13-7 CAS#:19287-45-7
CAS#:19287-45-7 CAS#:42976-02-3
CAS#:42976-02-3 CAS#:10544-50-0
CAS#:10544-50-0![[(EtH2N)B8H11NHEt] Structure](https://image.chemsrc.com/caspic/325/61289-01-8.png) CAS#:61289-01-8
CAS#:61289-01-8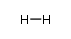 CAS#:1333-74-0
CAS#:1333-74-0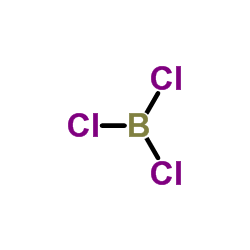 CAS#:10294-34-5
CAS#:10294-34-5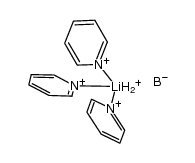 CAS#:244761-17-9
CAS#:244761-17-9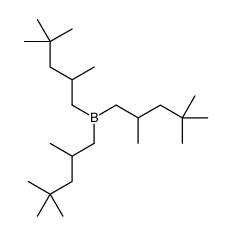 CAS#:14289-74-8
CAS#:14289-74-8 CAS#:446065-11-8
CAS#:446065-11-8 CAS#:1883-35-8
CAS#:1883-35-8 CAS#:23985-40-2
CAS#:23985-40-2 CAS#:121-43-7
CAS#:121-43-7 CAS#:10043-11-5
CAS#:10043-11-5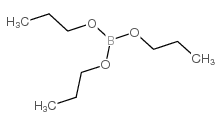 CAS#:688-71-1
CAS#:688-71-1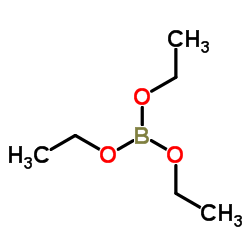 CAS#:150-46-9
CAS#:150-46-9
