2-Acetamido-1,2-dideoxynojirimycin
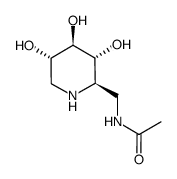
2-Acetamido-1,2-dideoxynojirimycin structure
|
Common Name | 2-Acetamido-1,2-dideoxynojirimycin | ||
|---|---|---|---|---|
| CAS Number | 105265-96-1 | Molecular Weight | 204.22400 | |
| Density | 1.35 g/cm3 | Boiling Point | 502.5ºC at 760 mmHg | |
| Molecular Formula | C8H16N2O4 | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 257.7ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Acetamido-1,2-dideoxynojirimycinAB05831, also known as 2-Acetamido-1,2-dideoxynojirimycin, is a highly potent and specific inhibitor of beta-hexosaminidase. N-Acetyl-3-hexosaminidase (HEX) is a member of lysosomal hydrolases, which catalyzes hydrolysis of terminal, non-reducing N-acetyl-|3-D-glucosamine (GlcNAc) andN-acetyl-(3-D-galactosamine (GalNAc) residues in glycoproteins, gan-gliosides, and glycosaminoglycans (GAGs). HEX, released by chondrocytes into the extracellular compartment, promotes cartilage matrix degradation. Osteoarthritis patients have increased HEX activity in synovial fluid. |
| Name | 2-acetamido-1,2,5-trideoxy-1,5-imino-d-g lucitol |
|---|---|
| Synonym | More Synonyms |
| Density | 1.35 g/cm3 |
|---|---|
| Boiling Point | 502.5ºC at 760 mmHg |
| Molecular Formula | C8H16N2O4 |
| Molecular Weight | 204.22400 |
| Flash Point | 257.7ºC |
| Exact Mass | 204.11100 |
| PSA | 101.82000 |
| Vapour Pressure | 3.33E-12mmHg at 25°C |
| Index of Refraction | 1.565 |
| InChIKey | GBRAQQUMMCVTAV-LXGUWJNJSA-N |
| SMILES | CC(=O)NC1CNC(CO)C(O)C1O |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | NONH for all modes of transport |
|
Bovine N-acetyl-beta-D-glucosaminidase: affinity purification and characterization of its active site with nitrogen containing analogs of N-acetylglucosamine.
Biochim. Biophys. Acta 1080 , 89-95, (1991) Two N-acetylglucosaminidases were isolated from bovine kidney with a three step procedure featuring affinity purification on 2-acetamido-1,2,5-trideoxy-1,5-iminoglucitol (2-acetamido-1,2-dideoxynojiri... |
|
|
Suppression of beta-N-acetylglucosaminidase in the N-glycosylation pathway for complex glycoprotein formation in Drosophila S2 cells.
Glycobiology 19 , 301-308, (2009) Most insect cells have a simple N-glycosylation process and consequently paucimannosidic or simple core glycans predominate. Previously, we have shown that paucimannosidic N-glycan structures are domi... |
|
|
Purification and characterization of recombinant human alpha-N-acetylglucosaminidase secreted by Chinese hamster ovary cells.
Protein Expr. Purif. 19 , 202-211, (2000) alpha-N-Acetylglucosaminidase (EC 3.2.1.50) is a lysosomal enzyme that is deficient in the genetic disorder Sanfilippo syndrome type B. To study the human enzyme, we expressed its cDNA in Lec1 mutant ... |
| 5-acetamido-1,3,4-thiadiazole |
| 2-acetamido-1,5-imino-1,2,5-trideoxy-D-glucitol |
| Acetamide,N-1,3,4-thiadiazol-2-yl |
| 2-acetamido-1,2,5-trideoxy-1,5-imino-D-glucitol |
| 2-acetylamino-1,3,4-thiadiazole |
| 1,3,4-Thiadiazole,2-acetamido |
| X 134 |
| 1,2-dideoxy-2-acetamidonojirimycin |
| N-Acetyl-2-amino-1,3,4-thiadiazol |
| 2-acetamido-1,2-dideoxynojirimycin |
 CAS#:105266-02-2
CAS#:105266-02-2 CAS#:114040-94-7
CAS#:114040-94-7 CAS#:50605-03-3
CAS#:50605-03-3 CAS#:117177-19-2
CAS#:117177-19-2 CAS#:130609-21-1
CAS#:130609-21-1 CAS#:153373-83-2
CAS#:153373-83-2