(1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol
![(1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol Structure](https://image.chemsrc.com/caspic/177/102225-88-7.png)
(1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol structure
|
Common Name | (1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol | ||
|---|---|---|---|---|
| CAS Number | 102225-88-7 | Molecular Weight | 248.40400 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C17H28O | Melting Point | 82-86ºC | |
| MSDS | Chinese USA | Flash Point | N/A | |
| Name | (1S)-1-[2,4,6-tri(propan-2-yl)phenyl]ethanol |
|---|---|
| Synonym | More Synonyms |
| Melting Point | 82-86ºC |
|---|---|
| Molecular Formula | C17H28O |
| Molecular Weight | 248.40400 |
| Exact Mass | 248.21400 |
| PSA | 20.23000 |
| LogP | 5.11010 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| Precursor 6 | |
|---|---|
| DownStream 4 | |
|
New asymmetric approach to natural pyrrolizidines: synthesis of (+)-amphorogynine A, (+)-amphorogynine D, and (+)-retronecine.
J. Org. Chem. 70 , 8352-8363, (2005) Three natural pyrrolizidines, (+)-amphorogynines A and D and (+)-retronecine, have been prepared from a common lactam intermediate. This central compound, in turn, was synthesized in diastereomericall... |
|
|
A New Asymmetric Carbon-Carbon Bond Forming Reaction: Four-Component Stereoselective Synthesis of (Z)-4,6-Dihydroxy-3-methylalk-2-enyl Methyl Sulfones This work was supported by the Swiss National Science Foundation.
Angew. Chem. Int. Ed. Engl. 39 , 1806, (2000)
|
|
|
Hamel, M., et al.
Tetrahedron Asymmetry 16 , 3406-3415, (2005)
|
| fs-0002 |
 CAS#:183202-00-8
CAS#:183202-00-8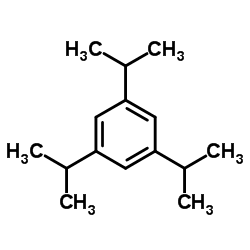 CAS#:717-74-8
CAS#:717-74-8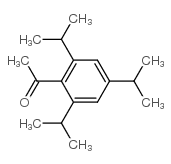 CAS#:2234-14-2
CAS#:2234-14-2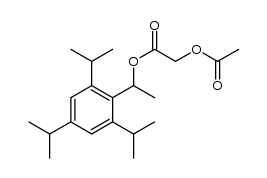 CAS#:1400563-23-6
CAS#:1400563-23-6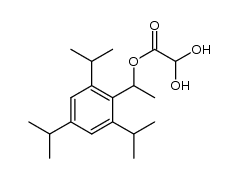 CAS#:1400563-22-5
CAS#:1400563-22-5![METHYL 8-CHLORO-6-(TRIFLUOROMETHYL)IMIDAZO[1,2-A]PYRIDINE-7-CARBOXYLATE structure](https://image.chemsrc.com/caspic/208/181531-14-6.png) CAS#:181531-14-6
CAS#:181531-14-6 CAS#:926622-52-8
CAS#:926622-52-8 CAS#:926622-53-9
CAS#:926622-53-9