149022-22-0
| Name | Amikacin sulfate salt |
|---|---|
| Synonyms |
AMIKACIN SULFATE 1:1.8
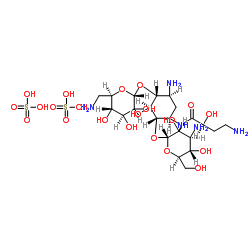
Butanamide, 4-amino-N-[(1R,2S,3S,4R,5S)-5-amino-2-[(3-amino-3-deoxy-α-D-glucopyranosyl)oxy]-4-[(6-amino-6-deoxy-α-D-glucopyranosyl)oxy]-3-hydroxycyclohexyl]-2-hydroxy-, (2S)-, sulfate (1:2) (s alt) (2S)-4-Amino-N-{(1R,2S,3S,4R,5S)-5-amino-2-[(3-amino-3-deoxy-α-D-glucopyranosyl)oxy]-4-[(6-amino-6-deoxy-α-D-glucopyranosyl)oxy]-3-hydroxycyclohexyl}-2-hydroxybutanamide sulfate (1:2) amikacin sulphate |
| Description | Amikacin sulfate (BAY 41-6551 sulfate) is an aminoglycoside antibiotic and a semisynthetic analog of kanamycin. Amikacin sulfate is bactericidal, acting directly on the 30S and 50S bacerial ribosomal subunits to inhibit protein synthesis. Amikacin sulfate is very active against most Gram-negative bacteria including gentamicin- and tobramycin-resistant strains. Amikacin sulfate also inhibits the infections caused by susceptible Nocardia and nontuberculous mycobacteria[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Amikacin offers definite advantages for treating infections caused by organisms resistant to other aminoglycosides. Amikaci is affected by relatively few arninoglycoside-modifying enzymes. Amikacin is useful in the treatment of infections caused by Nocardia asteroides, Mycobacterium avium-intracellulare, and certain species of "rapid-growing" mycobacteria (that is, M. chelonae and M. fortuitumi)[1]. Amikacin (100-1500 μM) causes a reliable dose-dependent loss of lateral line zebrafish hair cells with a LD50 value of 453 μM[3]. |
| In Vivo | Amikacin (320 mg/kg; subcutaneous injection; daily; for 10 days; male Fischer rats) treatment increases the chance of serious hearing loss in rats in vivo[3]. Animal Model: Male Fischer 344 rats (40-50-day-old)[3] Dosage: 320 mg/kg Administration: Subcutaneous injection; daily; for 10 days Result: Induced hearing loss in rats. |
| References |
[1]. Edson, R.S. and C.L. Terrell, The aminoglycosides. Mayo Clin Proc, 1999. 74(5): p. 519-28. [2]. Ristuccia AM, et al. An overview of amikacin. Ther Drug Monit. 1985;7(1):12-25. |
| Boiling Point | 981.8ºC at 760mmHg |
|---|---|
| Molecular Formula | C22H43N5O13.9/5H2O4S |
| Molecular Weight | 781.760 |
| Flash Point | 547.6°C |
| Exact Mass | 781.220520 |
| PSA | 497.90000 |
| Appearance | white to off-white |
| Vapour Pressure | 0mmHg at 25°C |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble50mg/mL |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | WK1961200 |
| HS Code | 2941902000 |
| HS Code | 2941902000 |
|---|