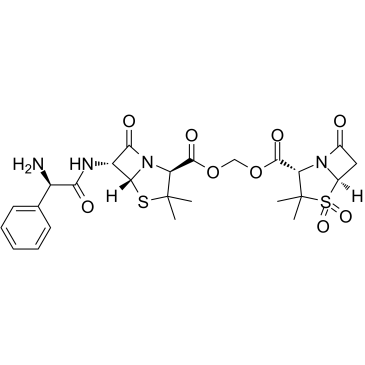
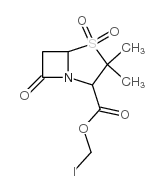
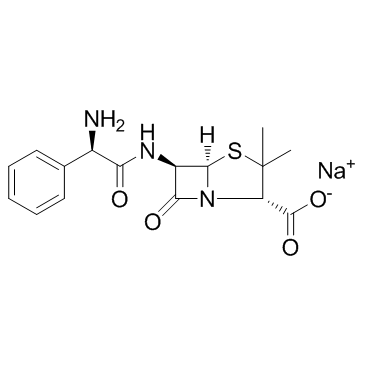
76497-13-7
| Name | sultamicillin |
|---|---|
| Synonyms |
6'-(2-Amino-2-phenylacetamido)penicillanoyloxymethylpenicillanate 1,1-dioxide
Sultancillin alkali Unacid PD SultaMcillin Base VD 1827 1,1-Dioxopenicillanoyloxymethyl 6-(D-a-amino-a-phenylacetamido)penicillanate ({[(2S,5R,6R)-6-{[(2R)-2-Amino-2-phenylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]hept-2-yl]carbonyl}oxy)methyl (2S,5R)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate 4,4-dioxide Sultamicillin Bethadl Orale Sultamicillin Base Baeimex |
| Description | Sultamicillin is an orally active double prodrug of Ampicillin/Sulbactan. Sulbactam is a semisynthetic beta-lactamase inhibitor which, in combination with Ampicillin, extends the antibacterial activity of the latter to include some beta-lactamase-producing strains of bacteria that would otherwise be resistant[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 907.7±65.0 °C at 760 mmHg |
| Melting Point | 190° |
| Molecular Formula | C25H30N4O9S2 |
| Molecular Weight | 594.657 |
| Flash Point | 502.8±34.3 °C |
| Exact Mass | 594.145447 |
| PSA | 216.16000 |
| LogP | -0.29 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.668 |
| Storage condition | 2-8°C |
| Water Solubility | Practically insoluble in water, very slightly soluble in methanol, practically insoluble in ethanol (96 per cent). |
| HS Code | 2934999090 |
|---|
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |