738-70-5
| Name | trimethoprim |
|---|---|
| Synonyms |
EINECS 212-006-2
Trimanyl Triprim Instalac Trimethoprim Monotrimin MFCD00036761 5-(3,4,5-trimethoxybenzyl)pyrimidine-2,4-diamine Trimogal 5-[(3,4,5-trimethoxyphenyl)methyl]pyrimidine-2,4-diamine WELLCOPRIM Proloprim Trimpex Monotrim TCMDC-125538 Uretrim Bactramin 5-(3,4,5-Trimethoxybenzyl)-2,4-pyrimidinediamine 5-{[3,4,5-tris(methyloxy)phenyl]methyl}pyrimidine-2,4-diamine Trimopan Syraprim Tiempe UNII-AN164J8Y0X |
| Description | Trimethoprim is a bacteriostatic antibiotic used mainly in the prophylaxis and treatment of urinary tract infections.Target: DHFRTrimethoprim (TMP), an inhibitor of dihydrofolate reductase, decreases the level of tetrahydrofolate supplying one-carbon units for biosynthesis of nucleotides, proteins, and panthotenate. TMP caused induction of DnaK, DnaJ, GroEL, ClpB, and IbpA/B Hsps. Among these Hsps, IbpA/B were most efficiently induced by TMP and coaggregated with the insoluble proteins [1]. Trimethoprim binds to dihydrofolate reductase and inhibits the reduction of dihydrofolic acid (DHF) to tetrahydrofolic acid (THF). THF is an essential precursor in the thymidine synthesis pathway and interference with this pathway inhibits bacterial DNA synthesis. Trimethoprim's affinity for bacterial dihydrofolate reductase is several thousand times greater than its affinity for human dihydrofolate reductase. Sulfamethoxazole inhibits dihydropteroate synthetase, an enzyme involved further upstream in the same pathway. Trimethoprim and sulfamethoxazole are commonly used in combination due to their synergistic effects. This drug combination also reduces the development of resistance that is seen when either drug is used alone [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 405.2±55.0 °C at 760 mmHg |
| Melting Point | 199-203 °C |
| Molecular Formula | C14H18N4O3 |
| Molecular Weight | 290.318 |
| Flash Point | 198.8±31.5 °C |
| Exact Mass | 290.137878 |
| PSA | 105.51000 |
| LogP | 0.38 |
| Vapour Pressure | 0.0±0.9 mmHg at 25°C |
| Index of Refraction | 1.600 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong oxidizing agents, acids. |
| Water Solubility | DMSO: soluble | <0.1 g/100 mL at 24 ºC |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | 3249 |
| WGK Germany | 3 |
| RTECS | UV8225000 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933599090 |
| Precursor 7 | |
|---|---|
| DownStream 4 | |
| HS Code | 2933599090 |
|---|---|
| Summary | 2933599090. other compounds containing a pyrimidine ring (whether or not hydrogenated) or piperazine ring in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |



![2,4-Dichloro-5-[3,4,5-trimethoxybenzyl]pyrimidine structure](https://image.chemsrc.com/caspic/088/55694-05-8.png)
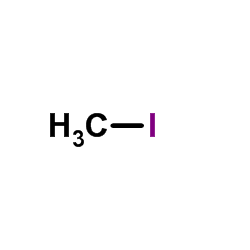
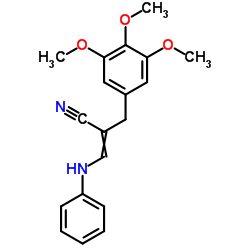

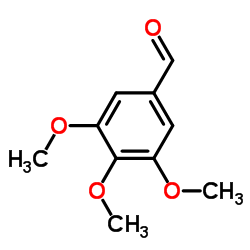

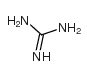

![N-[4-amino-5-(3,4,5-trimethoxy-benzyl)-pyrimidin-2-yl]-2-ethoxy-acetamide structure](https://image.chemsrc.com/caspic/343/68496-29-7.png)
