94805-82-0
| Name | glycycoumarin |
|---|---|
| Synonyms |
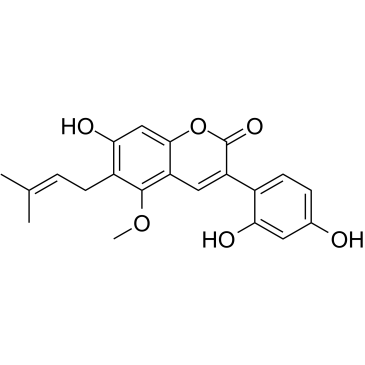
3-(2,4-Dihydroxy-phenyl)-7-hydroxy-5-methoxy-6-(3-methyl-but-2-enyl)-1-benzopyran-2-one
3-(2,4-Dihydroxyphenyl)-7-hydroxy-5-methoxy-6-(3-methyl-2-buten-1-yl)-2H-chromen-2-one glycycoumarin 3-(2,4-dihydroxyphenyl)-7-hydroxy-5-methoxy-6-(3-methylbut-2-enyl)chromen-2-one |
| Description | Glycycoumarin is a major bioactive coumarin of licorice. Glycycoumarin inhibits hepatocyte lipoapoptosis through activation of autophagy and inhibition of ER stress-mediated JNK and GSK-3-mediated mitochondrial pathway. Glycycoumarin exerts anti-liver cancer activity by directly targeting T-LAK cell-originated protein kinase [1] [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 646.9±55.0 °C at 760 mmHg |
| Melting Point | 243.5-244.5℃ |
| Molecular Formula | C21H20O6 |
| Molecular Weight | 368.380 |
| Flash Point | 232.0±25.0 °C |
| Exact Mass | 368.125977 |
| PSA | 100.13000 |
| LogP | 5.99 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.649 |
| Hazard Codes | Xi |
|---|