42438-89-1
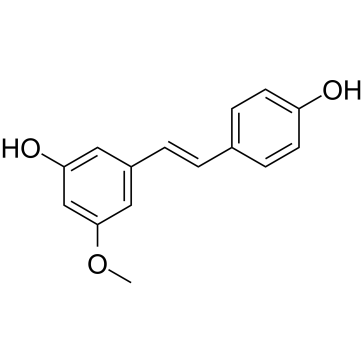
| Name | 3-[(E)-2-(4-hydroxyphenyl)ethenyl]-5-methoxyphenol |
|---|---|
| Synonyms |
mono-methylated resveratrol
3,4'-Dihydroxy-5-methoxy-trans-stilbene 3-[(E)-2-(4-Hydroxyphenyl)vinyl]-5-methoxyphenol Pinostilbene Pinostilbene hydrate 3-methoxyresveratrol trans-3,4'-dihydroxy-5-methoxystilbene phenol (3-[(E)-2-(4-hydroxyphenyl)ethenyl]-5-methoxy 5,4'-dihydroxy-3-methoxystilbene trans-Pinostilbene 3-methoxy-4',5-dihydroxy-trans-stilbene 4,3'-dihydroxy-5'-methoxystilbene |
| Description | Pinostilbene is a major metabolite of Pterostilbene. Pinostilbene exhibits inhibitory effects on colon cancer cells[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Pinostilbene (0- 40 μM; 24 hours, 48 hours) does not cause significant inhibition on the growth of normal colon cells[1]. Pinostilbene (20 μM, 40 μM; 24 hours, 48 hours) causes a significant and dose-dependent increase in the percentage of cells in S phase in both HCT116 and HT29 cells compared to the control cells[1]. Pinostilbene at μM also induces a modest increase of cell population in G2/M phase in HT29 cells[1]. Pinostilbene(20 μM, 40 μM; 24 hours, 48 hours) modulates expression of key signaling proteins related to cell proliferation and apoptosis[1]. Pinostilbene also acts as a resveratrol methylated derivative and displays protective effects against 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells[2]. Cell Viability Assay[1] Cell Line: HCT116 cells, HT29 cells Concentration: 24 hours, 48 hours Incubation Time: 0-100 μM Result: Inhibited the growth of two human colon cancer cells. Apoptosis Analysis[1] Cell Line: HCT116 cells, HT29 cells Concentration: 20 μM, 40 μM Incubation Time: 24 hours, 48 hours Result: Caused a significant and dose-dependent increase in the percentage of cells in S phase. Cell Viability Assay[1] Cell Line: HCT116 cells Concentration: 20 μM,40 μM Incubation Time: 24 hours, 48 hours Result: Significantly increased the expression levels of p53, Bax, cleaved caspase-3, cleaved PARP and p21Cip1/Waf1, while decreased the expression levels of cyclin E and p-Rb. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 454.3±33.0 °C at 760 mmHg |
| Melting Point | 117-118℃ |
| Molecular Formula | C15H14O3 |
| Molecular Weight | 242.270 |
| Flash Point | 228.5±25.4 °C |
| Exact Mass | 242.094299 |
| PSA | 49.69000 |
| LogP | 3.80 |
| Appearance | white to tan |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.692 |
| Storage condition | 2-8°C |
| Water Solubility | DMSO: ≥13mg/mL |
| Hazard Codes | Xi,N |
|---|---|
| Risk Phrases | 37/38-41-50 |
| Safety Phrases | 26-39-61 |
| RIDADR | UN 3077 9 / PGIII |
| HS Code | 2909500000 |
|
~% 
42438-89-1 |
| Literature: RUTGERS, THE STATE UNIVERSITY OF NEW JERSEY; THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF AGRICULTURE; MIZUNO, Cassia, Suemi Patent: WO2008/70872 A1, 2008 ; Location in patent: Page/Page column 28-29 ; |
|
~% 
42438-89-1 |
| Literature: Polunin, Konstantin E.; Polunina, Irina A.; Schmalz, Hans-Guenther Mendeleev Communications, 2002 , vol. 12, # 5 p. 178 - 180 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2909500000 |
|---|---|
| Summary | 2909500000 ether-phenols, ether-alcohol-phenols and their halogenated, sulphonated, nitrated or nitrosated derivatives VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |



![3-[2-(4-hydroxyphenyl)ethyl]-5-methoxyphenol structure](https://image.chemsrc.com/caspic/065/67884-29-1.png)