98319-26-7
| Name | finasteride |
|---|---|
| Synonyms |
Proscar
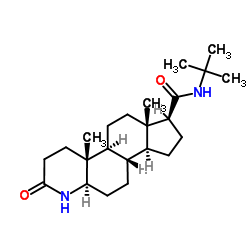
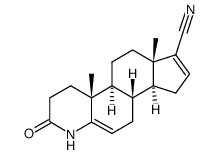
Finasteride (4aR,4bS,6aS,7S,9aS,9bS,11aR)-4a,6a-Dimethyl-N-(2-methyl-2-propanyl)-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide Prostide Finasterida (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-tert-Butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]quinoline-7-carboxamide 17b-(N-tert-Butylcarbamoyl)-4-aza-5a-androst-1-en-3-one (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-(1,1-diméthyléthyl)-4a,6a-diméthyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tétradécahydro-1H-indéno[5,4-f]quinoléine-7-carboxamide (5a,17b)-(1,1-Dimethylethyl)-3-oxo-4-azaandrost-1-ene-17-carboxamide MK-906 (4aR,4bS,6aS,7S,9aS,9bS,11aR)-2-Hydroxy-4a,6a-dimethyl-N-(2-methyl-2-propanyl)-4b,5,6,6a,7,8,9,9a,9b,10,11,11a-dodecahydro-4aH-indeno[5,4-f]quinoline-7-carboximidic acid Chibro-Proscar Finastid Propecia Finpecia (4aR,4bS,6aS,7S,9aS,9bS,11aR)-N-tert-Butyl-4a,6a-dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-indeno[5,4-f]chinolin-7-carboxamid Propecia (TN) Proscar (TN) (1S,3aS,3bS,5aR,9aR,9bS,11aS)-N-tert-butyl-9a,11a-dimethyl-7-oxo-1,2,3,3a,3b,4,5,5a,6,9b,10,11-dodecahydroindeno[5,4-f]quinoline-1-carboxamide MFCD00869737 Finasteridum |
| Description | Finasteride is an orally active testosterone 5-alpha-reductase inhibitor (Ki= 10 nM). Target: 5-alpha ReductaseApproved: 1992Finasteride, a synthetic 4-azasteroid antiandrogen compound, is a specific inhibitor of steroid Type II 5α-reductase, an intracellular enzyme that converts the androgen testosterone into 5α-dihydrotestosterone (DHT). Finasteride is used in the treatment of prostate cancer, benign prostatic hyperplasia, and androgenetic alopecia (male pattern baldness). In benign prostatic hyperplasia, finasteride inhibits 5alpha-reductase activity in epithelium for Ki of 10 nM, significantly lower than in stroma (Ki = 33nM) [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 576.6±50.0 °C at 760 mmHg |
| Melting Point | 253 °C |
| Molecular Formula | C23H36N2O2 |
| Molecular Weight | 372.544 |
| Flash Point | 177.4±30.3 °C |
| Exact Mass | 372.277679 |
| PSA | 58.20000 |
| LogP | 3.24 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.524 |
| Storage condition | Store at RT |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Faceshields;Gloves |
| Hazard Codes | T:Toxic |
| Risk Phrases | R22;R60;R61 |
| Safety Phrases | S36/37/39-S45-S53 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CL5245000 |
| HS Code | 2937290090 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
| HS Code | 2937290090 |
|---|