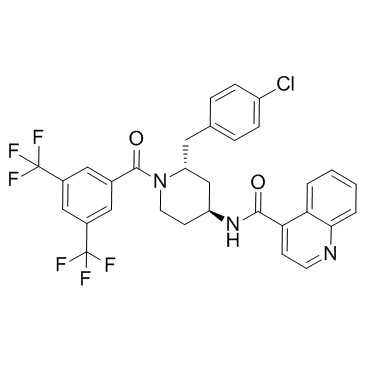
177707-12-9
| Name | N-[(2R,4S)-1-[3,5-bis(trifluoromethyl)benzoyl]-2-[(4-chlorophenyl)methyl]piperidin-4-yl]quinoline-4-carboxamide |
|---|---|
| Synonyms |
nk-608
nkp608||nk-608 N-[(2R,4S)-1-[3,5-Bis(trifluoromethyl)benzoyl]-2-(4-chlorobenzyl)-4-piperidinyl]-4-quinolinecarboxamide T66 BNJ EVM- DT6NTJ AVR CXFFF EXFFF& B1R DG &&(2R)-trans Form nkp608 N-[(2R,4S)-1-[3,5-Bis(trifluoromethyl)benzoyl]-2-[(4-chlorophenyl)methyl]-4-piperidinyl]-4-quinolinecarboxamide cs-1077 N-[(2R,4S)-1-[3,5-Bis(trifluoromethyl)benzoyl]-2-(4-chlorobenzyl)piperidin-4-yl]quinoline-4-carboxamide |
| Description | NKP608 is a non-peptidic derivative of 4-aminopiperidine which acts as a selective, specific and potent antagonist at the neurokinin-1 (NK-1) receptor both in vitro(IC50=2.6 nM) and in vivo. IC50 value: 2.6 nMTarget: NK-1 receptorIn vitro, the binding of NKP608 to bovine retina was characterized by an IC50 of 2.6+/-0.4 nM, whereas the compound's affinity to other receptor binding sites, including NK-2 and NK-3, was much lower. Species differences in IC(50) values with NKP608 were less pronounced than with previously described NK-1 receptor antagonists, being 13+/-2 and 27+/-2 nM in gerbil midbrain and rat striatum, respectively. In vivo, using the hind foot thumping model in gerbils, NKP608 exhibited a potent NK-1 antagonistic activity following oral administration (ID(50)=0.23 mg/kg; 2 h pretreatment), supporting a central activity of NKP608. NKP608 may prove a useful anxiolytic compound. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 719.2±60.0 °C at 760 mmHg |
| Molecular Formula | C31H24ClF6N3O2 |
| Molecular Weight | 619.985 |
| Flash Point | 388.8±32.9 °C |
| Exact Mass | 619.146118 |
| PSA | 62.30000 |
| LogP | 6.74 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.614 |
| Storage condition | 2-8℃ |