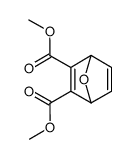
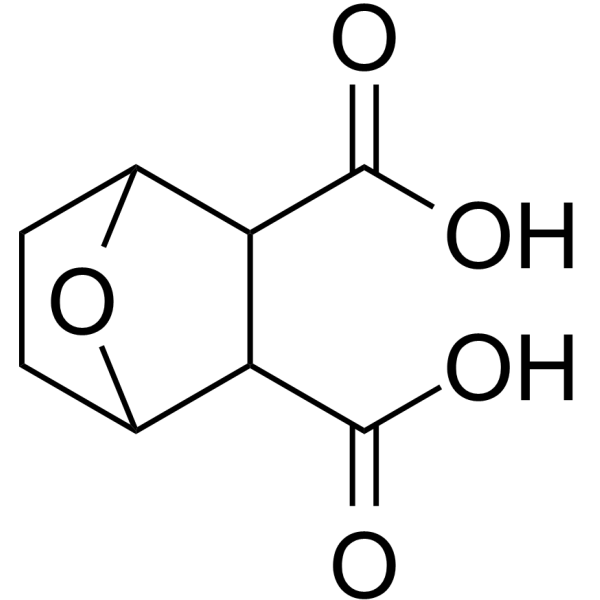
145-73-3
| Name | endothal |
|---|---|
| Synonyms |
aquatholplus
7-oxa-bicyclo[2.2.1]heptane-2,3-dicarboxylic acid 7-Oxabicyclo[2.2.1]heptane-2,3-dicarboxylicacid Aquathol Hydout 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid ENDOTHAL ACCELERATE MFCD00137362 Niagrathal Hydrothol Hydrothal EINECS 205-660-5 (1Ξ,2Ξ,3Ξ,4Ξ)-7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid ENDOTHALL Emdothal |
| Description | Endothall (Endothal) is a protein phosphatase 2A (PP2A) inhibitor with IC50s of 90 nM and 5 µM for PP2A and PP1, respectively. Endothall can be used as an herbicide. Endothall also is useful in cancer chemotherapy[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Endothall, an organic acid, is the least toxic structural analogue of Cantharidin that still inhibits PP2A[2]. Endothall inhibits preferentially hepatocellular carcinoma (HCC) growth and these new rat hepatocellular carcinoma lines may be useful for further biochemical and pharmacological studies on PP2A inhibitors, and for testing new forms of treatment of hepatic cell carcinomas. The HR-2, HR-3, HR-4, and Zajdela hepatocellular carcinomas are most sensitive to Endothall (IC50 of 1.7, 1.2, 0.9, and 1.7 µg/mL), whereas newborn rat hepatocytes growing exponentially in primary culture (IC50=6.2 µg/mL), rat DHD/K12 colon carcinoma cells (IC50=3.6 µg/mL), or human HT-29 colon carcinoma cells (IC50=4.9 µg/mL) were less sensitive[2]. Endothall inhibits the growth of HCC lines in culture more than that of normal hepatocytes or colon carcinomas, inducing mitotic arrest, followed by cell death. Endothall causes dose- and time-dependent cytostasis specifically in G2/M[2]. Endothall (3 µg/mL) inhibits the cell cycle at G2/M and subsequent apoptotic cell death[2]. |
| In Vivo | Endothall exhibits acute LD50 of 14 mg/kg in mice[2]. |
| References |
| Density | 1.431 |
|---|---|
| Boiling Point | 350ºC(e) |
| Melting Point | 144ºC |
| Molecular Formula | C8H10O5 |
| Molecular Weight | 186.16200 |
| Flash Point | 190.5ºC |
| Exact Mass | 186.05300 |
| PSA | 83.83000 |
| Vapour Pressure | 2.88E-09mmHg at 25°C |
| Index of Refraction | 1.568 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T |
|---|---|
| Risk Phrases | 21-25-36/37/38 |
| Safety Phrases | S45;S36/S37/S39 |
| RIDADR | UN 2811 6.1/PG 2 |
| WGK Germany | 1 |
| RTECS | RN7875000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2932190013 |
|
~% 
145-73-3 |
| Literature: Zeitschrift fuer Chemie (Stuttgart, Germany), , vol. 24, # 11 p. 405 - 406 |
|
~% 
145-73-3 |
| Literature: Zeitschrift fuer Chemie (Stuttgart, Germany), , vol. 24, # 11 p. 405 - 406 |
|
~% 
145-73-3 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 535, p. 113,120 Journal fuer Praktische Chemie (Leipzig), , vol. <2> 156, p. 285,305 |
|
~% 
145-73-3 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 535, p. 113,120 |
|
~% 
145-73-3 |
| Literature: Journal fuer Praktische Chemie (Leipzig), , vol. <2> 156, p. 285,305 |
| HS Code | 2932190013 |
|---|---|
| Summary | 2932190013 7-oxabicyclo[2.2.1]heptane-2,3-dicarboxylic acid。supervision conditions:s(import or export registration certificate for pesticides)。VAT:17.0%。tax rebate rate:9.0%。MFN tarrif:6.5%。general tariff:20.0% |